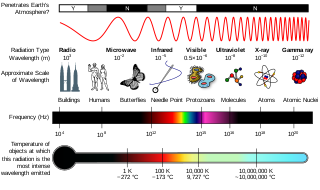
The electromagnetic spectrum is the full range of electromagnetic radiation, organized by frequency or wavelength. The spectrum is divided into separate bands, with different names for the electromagnetic waves within each band. From low to high frequency these are: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. The electromagnetic waves in each of these bands have different characteristics, such as how they are produced, how they interact with matter, and their practical applications.

Fluorescence is one of two kinds of emission of light by a substance that has absorbed light or other electromagnetic radiation. Fluorescence involves no change in electron spin multiplicity and generally it immediately follows absorption; phosphorescence involves spin change and is delayed. Thus fluorescent materials generally cease to glow nearly immediately when the radiation source stops, while phosphorescent materials, which continue to emit light for some time after.

A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light is determined by the energy required for electrons to cross the band gap of the semiconductor. White light is obtained by using multiple semiconductors or a layer of light-emitting phosphor on the semiconductor device.

Ultraviolet (UV) light is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs, Cherenkov radiation, and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights.
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or visible light, and cathodoluminescent substances which glow when struck by an electron beam in a cathode-ray tube.

A fluorescent lamp, or fluorescent tube, is a low-pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor, which produces short-wave ultraviolet light that then causes a phosphor coating on the inside of the lamp to glow. A fluorescent lamp converts electrical energy into useful light much more efficiently than an incandescent lamp. The typical luminous efficacy of fluorescent lighting systems is 50–100 lumens per watt, several times the efficacy of incandescent bulbs with comparable light output. For comparison, the luminous efficacy of an incandescent bulb may only be 16 lumens per watt.

Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluorescence, a phosphorescent material does not immediately reemit the radiation it absorbs. Instead, a phosphorescent material absorbs some of the radiation energy and reemits it for a much longer time after the radiation source is removed.

A blacklight, also called a UV-A light, Wood's lamp, or ultraviolet light, is a lamp that emits long-wave (UV-A) ultraviolet light and very little visible light. One type of lamp has a violet filter material, either on the bulb or in a separate glass filter in the lamp housing, which blocks most visible light and allows through UV, so the lamp has a dim violet glow when operating. Blacklight lamps which have this filter have a lighting industry designation that includes the letters "BLB". This stands for "blacklight blue". A second type of lamp produces ultraviolet but does not have the filter material, so it produces more visible light and has a blue color when operating. These tubes are made for use in "bug zapper" insect traps, and are identified by the industry designation "BL". This stands for "blacklight".

A fluorophore is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds.

A safelight is a light source suitable for use in a photographic darkroom. It provides illumination only from parts of the visible spectrum to which the photographic material in use is nearly, or completely insensitive.

A mercury-vapor lamp is a gas-discharge lamp that uses an electric arc through vaporized mercury to produce light. The arc discharge is generally confined to a small fused quartz arc tube mounted within a larger soda lime or borosilicate glass bulb. The outer bulb may be clear or coated with a phosphor; in either case, the outer bulb provides thermal insulation, protection from the ultraviolet radiation the light produces, and a convenient mounting for the fused quartz arc tube.

A germicidal lamp is an electric light that produces ultraviolet C (UVC) light. This short-wave ultraviolet light disrupts DNA base pairing, causing formation of pyrimidine dimers, and leads to the inactivation of bacteria, viruses, and protozoans. It can also be used to produce ozone for water disinfection. They are used in ultraviolet germicidal irradiation (UVGI).

The induction lamp, electrodeless lamp, or electrodeless induction lamp is a gas-discharge lamp in which an electric or magnetic field transfers the power required to generate light from outside the lamp envelope to the gas inside. This is in contrast to a typical gas discharge lamp that uses internal electrodes connected to the power supply by conductors that pass through the lamp envelope. Eliminating the internal electrodes provides two advantages:

Yttrium aluminium garnet (YAG, Y3Al5O12) is a synthetic crystalline material of the garnet group. It is a cubic yttrium aluminium oxide phase, with other examples being YAlO3 (YAP) in a hexagonal or an orthorhombic, perovskite-like form, and the monoclinic Y4Al2O9 (YAM).
Luminous paint is paint that emits visible light through fluorescence, phosphorescence, or radioluminescence.

Strontium aluminate is an aluminate compound with the chemical formula SrAl2O4. It is a pale yellow, monoclinic crystalline powder that is odourless and non-flammable. When activated with a suitable dopant, it acts as a photoluminescent phosphor with long persistence of phosphorescence.

Ultraviolet photography is a photographic process of recording images by using radiation from the ultraviolet (UV) spectrum only. Images taken with ultraviolet radiation serve a number of scientific, medical or artistic purposes. Images may reveal deterioration of art works or structures not apparent under light. Diagnostic medical images may be used to detect certain skin disorders or as evidence of injury. Some animals, particularly insects, use ultraviolet wavelengths for vision; ultraviolet photography can help investigate the markings of plants that attract insects, while invisible to the unaided human eye. Ultraviolet photography of archaeological sites may reveal artifacts or traffic patterns not otherwise visible.

An ultraviolet (UV) marker is a pen whose marks are fluorescent but transparent; the marks can be seen only under an ultraviolet light. They are commonly used in security situations to identify belongings or to prevent the reproduction of unauthorized banknotes. UV pens can now be bought at some stationery shops to securely mark items of high value in case of theft.

Super-LumiNova is a brand name under which strontium aluminate–based non-radioactive and nontoxic photoluminescent or afterglow pigments for illuminating markings on watch dials, hands and bezels, etc. in the dark are marketed. When activated with a suitable dopant, it acts as a photoluminescent phosphor with long persistence of phosphorescence. This technology offers up to ten times higher brightness than previous zinc sulfide–based materials.
Kaede is a photoactivatable fluorescent protein naturally originated from a stony coral, Trachyphyllia geoffroyi. Its name means "maple" in Japanese. With the irradiation of ultraviolet light (350–400 nm), Kaede undergoes irreversible photoconversion from green fluorescence to red fluorescence.




















