| |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.051.194 |
| Chemical and physical data | |
| Formula | C24H23N3O6S |
| Molar mass | 481.52 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
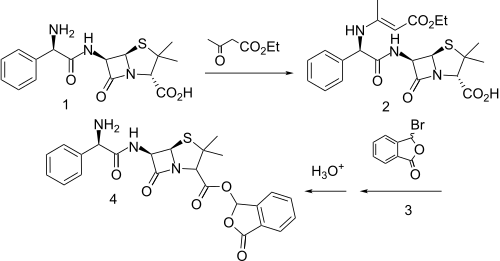
Talampicillin is a beta lactam antibiotic from the penicillin family. It is an acid stable prodrug that was administered orally. It is not approved by the FDA for use in the United States. It should be avoided in Liver diseases