
A carboxylic acid is an organic acid that contains a carboxyl group (C(=O)OH) attached to an R-group. The general formula of a carboxylic acid is R−COOH or R−CO2H, with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

Kevlar (para-aramid) is a heat-resistant and strong synthetic fiber, related to other aramids such as Nomex and Technora. Developed by Stephanie Kwolek at DuPont in 1965, the high-strength material was first used commercially in the early 1970s as a replacement for steel in racing tires. It is typically spun into ropes or fabric sheets that can be used as such, or as an ingredient in composite material components.
In chemistry, a monomer is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Aramid fibers, short for aromatic polyamide, are a class of heat-resistant and strong synthetic fibers. They are used in aerospace and military applications, for ballistic-rated body armor fabric and ballistic composites, in marine cordage, marine hull reinforcement, and as an asbestos substitute.
A polyamide is a polymer with repeating units linked by amide bonds.
Twaron is a para-aramid. It is a heat-resistant and strong synthetic fibre developed in the early 1970s by the Dutch company Akzo Nobel's division Enka BV, later Akzo Industrial Fibers. The research name of the para-aramid fibre was originally Fiber X, but it was soon called Arenka. Although the Dutch para-aramid fiber was developed only a little later than DuPont's Kevlar, the introduction of Twaron as a commercial product came much later than Kevlar due to financial problems at the AKZO company in the 1970s.

p-Phenylenediamine (PPD) is an organic compound with the formula C6H4(NH2)2. This derivative of aniline is a white solid, but samples can darken due to air oxidation. It is mainly used as a component of engineering polymers and composites like kevlar. It is also an ingredient in hair dyes and is occasionally used as a substitute for henna.
Polymer chemistry is a sub-discipline of chemistry that focuses on the chemical synthesis, structure, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are also applicable through a wide range of other chemistry sub-disciplines like organic chemistry, analytical chemistry, and physical chemistry. Many materials have polymeric structures, from fully inorganic metals and ceramics to DNA and other biological molecules, however, polymer chemistry is typically referred to in the context of synthetic, organic compositions. Synthetic polymers are ubiquitous in commercial materials and products in everyday use, commonly referred to as plastics, and rubbers, and are major components of composite materials. Polymer chemistry can also be included in the broader fields of polymer science or even nanotechnology, both of which can be described as encompassing polymer physics and polymer engineering.

Nomex is a flame-resistant meta-aramid material developed in the early 1960s by DuPont and first marketed in 1967.

In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure C6H5CH2–. Benzyl features a benzene ring attached to a CH2 group.

Terephthalic acid is an organic compound with formula C6H4(CO2H)2. This white solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several million tonnes are produced annually. The common name is derived from the turpentine-producing tree Pistacia terebinthus and phthalic acid.
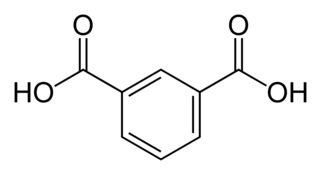
Isophthalic acid is an organic compound with the formula C6H4(CO2H)2. This colorless solid is an isomer of phthalic acid and terephthalic acid. The main industrial uses of purified isophthalic acid (PIA) are for the production of polyethylene terephthalate (PET) resin and for the production of unsaturated polyester resin (UPR) and other types of coating resins.

A sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.
Polybenzimidazole fiber is a synthetic fiber with a very high decomposition temperature and doesn't exhibit a melting point. It has exceptional thermal and chemical stability and does not readily ignite. It was first discovered by American polymer chemist Carl Shipp Marvel in the pursuit of new materials with superior stability, retention of stiffness, toughness at elevated temperature. Due to its high stability, polybenzimidazole is used to fabricate high-performance protective apparel such as firefighter's gear, astronaut space suits, high temperature protective gloves, welders’ apparel and aircraft wall fabrics. Polybenzimidazole has been applied as a membrane in fuel cells.
Polysulfones are a family of high performance thermoplastics. These polymers are known for their toughness and stability at high temperatures. Technically used polysulfones contain an aryl-SO2-aryl subunit. Due to the high cost of raw materials and processing, polysulfones are used in specialty applications and often are a superior replacement for polycarbonates.

Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.
A repeat unit or repeating unit is a part of a polymer whose repetition would produce the complete polymer chain by linking the repeat units together successively along the chain, like the beads of a necklace.
Technora is an aramid that is useful for a variety of applications that require high strength or chemical resistance. It is a brand name of the company Teijin Aramid.

Tetramethylurea is the organic compound with the formula (Me2N)2CO. It is a substituted urea. This colorless liquid is used as an aprotic-polar solvent, especially for aromatic compounds and is used e. g. for Grignard reagents.
1,4-Bis(trichloromethyl)benzene is an organic compound with the formula C6H4(CCl3)2. A white solid, it is prepared industrially by chlorination of para-xylene. It reacts with terephthalic acid to give terephthaloyl chloride, a precursor to Kevlar. It also reacts with sulfur dioxide to give the same acid chloride and thionyl chloride. It reacts with hydrogen fluoride in 1,2-dichloroethane to form 1,4-bis(chlorodifluoromethyl)benzene in a yield of 79%.












