
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen or sulfur. Rarer still, they may contain elements such as phosphorus, chlorine, and bromine.

Batrachotoxin (BTX) is an extremely potent cardiotoxic and neurotoxic steroidal alkaloid found in certain species of beetles, birds, and frogs. The name is from the Greek word βάτραχος, bátrachos, 'frog'. Structurally-related chemical compounds are often referred to collectively as batrachotoxins. In certain frogs, this alkaloid is present mostly on the skin. Such frogs are among those used for poisoning darts. Batrachotoxin binds to and irreversibly opens the sodium channels of nerve cells and prevents them from closing, resulting in paralysis and death. No antidote is known.

Phytochemistry is the study of phytochemicals, which are chemicals derived from plants. Phytochemists strive to describe the structures of the large number of secondary metabolites found in plants, the functions of these compounds in human and plant biology, and the biosynthesis of these compounds. Plants synthesize phytochemicals for many reasons, including to protect themselves against insect attacks and plant diseases. The compounds found in plants are of many kinds, but most can be grouped into four major biosynthetic classes: alkaloids, phenylpropanoids, polyketides, and terpenoids.
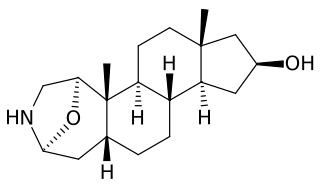
Samandarin or Samandarine is the main steroidal alkaloid secreted by the fire salamander (Salamandra salamandra). The compound is extremely toxic (LD50 = 70 μg/kg in mice). Poisoning can cause convulsions, respiratory paralysis, and eventual death. Samandarin is also believed to be the active ingredient in Salamander brandy, a Slovenian traditional medicinal alcoholic drink with purported hallucinogenic and aphrodisiac effects.

Cyclopamine (11-deoxojervine) is a naturally occurring steroidal alkaloid. It is a teratogenic component of corn lily, which when consumed during gestation has been demonstrated to induce birth defects, including the development of a single eye (cyclopia) in offspring. The molecule was named after this effect, which was originally observed by Idaho lamb farmers in 1957 after their herds gave birth to cycloptic lambs. It then took more than a decade to identify corn lily as the culprit. Later work suggested that differing rain patterns had changed grazing behaviours, which led to a greater quantity of corn lily to be ingested by pregnant sheep. Cyclopamine interrupts the sonic hedgehog signalling pathway, instrumental in early development, ultimately causing birth defects.

Veratridine is a steroidal alkaloid found in plants of the lily family, specifically the genera Veratrum and Schoenocaulon. Upon absorption through the skin or mucous membranes, it acts as a neurotoxin by binding to and preventing the inactivation of voltage-gated sodium ion channels in heart, nerve, and skeletal muscle cell membranes. Veratridine increases nerve excitability and intracellular Ca2+ concentrations.

Asparagus racemosus is a species of asparagus native from Africa through southern Asia, including the Indian subcontinent, to northern Australia. It grows 1–2 m tall and prefers to take root in gravelly, rocky soils high up in piedmont plains, at 1,300–1,400 m (4,300–4,600 ft) elevation. It was botanically described in 1799. Because of its multiple uses, the demand for Asparagus racemosus is constantly on the rise. Due to destructive harvesting, combined with habitat destruction, and deforestation, the plant is now considered "endangered" in its natural habitat.

Buxus sempervirens, the common box, European box, or boxwood, is a species of flowering plant in the genus Buxus, native to western and southern Europe, northwest Africa, and southwest Asia, from southern England south to northern Morocco, and east through the northern Mediterranean region to Turkey. Buxus colchica of western Caucasus and B. hyrcana of northern Iran and eastern Caucasus are commonly treated as synonyms of B. sempervirens.

Conessine is a steroidal alkaloid found in a number of plant species from the family Apocynaceae, including Holarrhena floribunda, Holarrhena antidysenterica and Funtumia elastica. It acts as a histamine antagonist, selective for the H3 subtype (with an affinity of pKi = 8.27; Ki = ~5 nM). It was also found to have long CNS clearance times, high blood–brain barrier penetration and high affinity for the adrenergic receptors.

Tomatine is a glycoalkaloid, found in the stems and leaves of tomato plants, and in the fruits at much lower concentrations. Chemically pure tomatine is a white crystalline solid at standard temperature and pressure.

Solamargine is a cytotoxic chemical compound that occurs in plants of the family Solanaceae, such as potatoes, tomatoes, and eggplants. It has been also isolated from Solanum nigrum fungal endophyte Aspergillus flavus. It is a glycoalkaloid derived from the steroidal alkaloid solasodine.
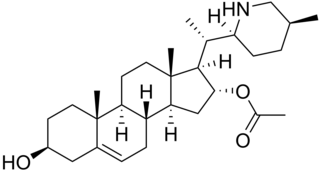
Muldamine is a phytosterol alkaloid isolated from Veratrum californicum. It is the acetate ester of the piperidine steroid teinemine.

The cortistatins are a group of steroidal alkaloids first isolated in 2006 from the marine sponge Corticium simplex. The cortistatins were first discovered in a search for naturally occurring compounds that inhibit proliferation of human umbilical vein endothelial cells (HUVECs), with cortistatin A being the most potent compound in the class.

Solanidine is a poisonous steroidal alkaloid chemical compound that occurs in plants of the family Solanaceae, such as potato and Solanum americanum. The sugar portion of glycoalkaloids hydrolyses in the body, leaving the solanidine portion.

Steroidal alkaloids have the basic steroidal skeleton with nitrogen-based functional groups attached to the skeleton. More specifically, they are distinguished by their tetracyclic cyclopentanoperhydrophenanthrene skeleton that marks their close relationship with sterols. They fall in two major categories: Solanum alkaloids and Veratrum alkaloids. A Steroidal alkaloid has also been found in Chonemorpha fragrans, 'chonemorphine' was used to treat intestinal infections in Wistar rats..
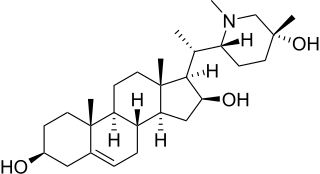
Pingbeinine is a steroidal alkaloid isolated from Fritillaria.
Biomimetic synthesis is an area of organic chemical synthesis that is specifically biologically inspired. The term encompasses both the testing of a "biogenetic hypothesis" through execution of a series of reactions designed to parallel the proposed biosynthesis, as well as programs of study where a synthetic reaction or reactions aimed at a desired synthetic goal are designed to mimic one or more known enzymic transformations of an established biosynthetic pathway. The earliest generally cited example of a biomimetic synthesis is Sir Robert Robinson's organic synthesis of the alkaloid tropinone.
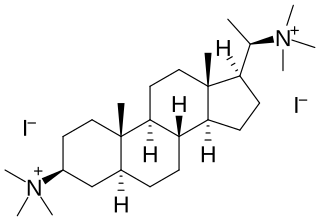
Malouetine is an aminosteroid neuromuscular blocking agent and antinicotinic alkaloid isolated from Malouetia spp.

Sarcococca saligna, the sweet box or Christmas box, is a species of flowering plant in the family Buxaceae. This shrub is native to northern Pakistan. Its common name in Pakistan is sheha.

Alma Levant Hayden was an American chemist, and one of the first African-American women to gain a scientist position at a science agency in Washington, D.C. She joined the National Institutes of Health (NIH) in the 1950s. Hayden graduated from Howard University with a master's degree in chemistry, and became an expert in spectrophotometry, the measurement of how substances absorb light. She published work on infrared and other techniques for analyzing chemicals in a range of journals. Hayden was appointed Chief of the Spectrophotometer Research Branch in the Division of Pharmaceutical Chemistry at the U.S. Food and Drug Administration (FDA) in 1963, and may have been the first African-American scientist at the FDA. Hayden came to national attention in 1963 when she led the team that exposed the common substance in Krebiozen, a long-controversial alternative and expensive drug promoted as anti-cancer.


















