Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bottles, etc.). As of 2017, over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market.

In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile.

In organic chemistry, the ene reaction is a chemical reaction between an alkene with an allylic hydrogen and a compound containing a multiple bond, in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.

Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak base; its conjugate acid has a pKa of 0.8, compared to 7 for imidazole.

tert-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as t-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. tert-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether.

In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group. If the R′ is hydrogen, the compounds are called hydroperoxides, which are discussed in that article. The O−O bond of peroxides easily breaks, producing free radicals of the form RO•. Thus, organic peroxides are useful as initiators for some types of polymerization, such as the acrylic, unsaturated polyester, and vinyl ester resins used in glass-reinforced plastics. MEKP and benzoyl peroxide are commonly used for this purpose. However, the same property also means that organic peroxides can explosively combust. Organic peroxides, like their inorganic counterparts, are often powerful bleaching agents.
Autoxidation refers to oxidations brought about by reactions with oxygen at normal temperatures, without the intervention of flame or electric spark. The term is usually used to describe the gradual degradation of organic compounds in air at ambient temperatures. Many common phenomena can be attributed to autoxidation, such as food going rancid, the 'drying' of varnishes and paints, and the perishing of rubber. It is also an important concept in both industrial chemistry and biology. Autoxidation is therefore a fairly broad term and can encompass examples of photooxygenation and catalytic oxidation.

Hydroperoxides or peroxols are compounds of the form ROOH, which contain the hydroperoxy functional group (–OOH). The hydroperoxide anion and the neutral hydroperoxyl radical (HOO·) consist of an unbond hydroperoxy group. When R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. Organic hydroperoxides can either intentionally or unintentionally initiate explosive polymerisation in materials with unsaturated chemical bonds.

The Wharton olefin synthesis or the Wharton reaction is a chemical reaction that involves the reduction of α,β-epoxy ketones using hydrazine to give allylic alcohols. This reaction, introduced in 1961 by P. S. Wharton, is an extension of the Wolff–Kishner reduction. The general features of this synthesis are: 1) the epoxidation of α,β-unsaturated ketones is achieved usually in basic conditions using hydrogen peroxide solution in high yield; 2) the epoxy ketone is treated with 2–3 equivalents of a hydrazine hydrate in presence of substoichiometric amounts of acetic acid. This reaction occurs rapidly at room temperature with the evolution of nitrogen and the formation of an allylic alcohol. It can be used to synthesize carenol compounds. Wharton's initial procedure has been improved.

The Dakin oxidation (or Dakin reaction) is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde (2-hydroxybenzaldehyde or 4-hydroxybenzaldehyde) or ketone reacts with hydrogen peroxide (H2O2) in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidised, whereas the H2O2 is reduced.

Di-tert-butyl peroxide or DTBP is an organic compound consisting of a peroxide group bonded to two tert-butyl groups. It is one of the most stable organic peroxides, due to the tert-butyl groups being bulky. It is a colorless liquid.
Selenoxide elimination is a method for the chemical synthesis of alkenes from selenoxides. It is most commonly used to synthesize α,β-unsaturated carbonyl compounds from the corresponding saturated analogues. It is mechanistically related to the Cope reaction.
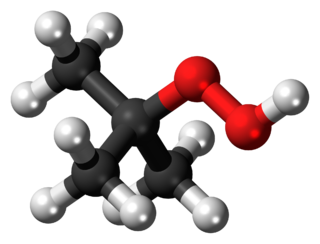
tert-Butyl hydroperoxide (tBuOOH) is the organic compound with the formula (CH3)3COOH. It is one of the most widely used hydroperoxides in a variety of oxidation processes, for example the Halcon process. It is normally supplied as a 69–70% aqueous solution. Compared to hydrogen peroxide and organic peracids, tert-butyl hydroperoxide is less reactive and more soluble in organic solvents. Overall, it is renowned for the convenient handling properties of its solutions. Its solutions in organic solvents are highly stable.

Oxazoline is a five-membered heterocyclic organic compound with the formula C3H5NO. It is the parent of a family of compounds called oxazolines, which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond.

Dichlorotris(triphenylphosphine)ruthenium(II) is a coordination complex of ruthenium. It is a chocolate brown solid that is soluble in organic solvents such as benzene. The compound is used as a precursor to other complexes including those used in homogeneous catalysis.
The Kharasch–Sosnovsky reaction is the radical oxidation of an allylic alkene to a allylic alcohol using a copper catalyst and a peroxy ester or a peroxide. Chiral ligands can be used to render the reaction asymmetric, constructing chiral C–O bonds via C–H bond activation. This is notable as asymmetric addition to allylic groups tends to be difficult due to the transition state being highly symmetric. The reaction is named after Morris S. Kharasch and George Sosnovsky who first reported it in 1958.

Trifluoroperacetic acid is an organofluorine compound, the peroxy acid analog of trifluoroacetic acid, with the condensed structural formula CF
3COOOH. It is a strong oxidizing agent for organic oxidation reactions, such as in Baeyer–Villiger oxidations of ketones. It is the most reactive of the organic peroxy acids, allowing it to successfully oxidise relatively unreactive alkenes to epoxides where other peroxy acids are ineffective. It can also oxidise the chalcogens in some functional groups, such as by transforming selenoethers to selones. It is a potentially explosive material and is not commercially available, but it can be quickly prepared as needed. Its use as a laboratory reagent was pioneered and developed by William D. Emmons.
The Mukaiyama hydration is an organic reaction involving formal addition of an equivalent of water across an olefin by the action of catalytic bis(acetylacetonato)cobalt(II) complex, phenylsilane and atmospheric oxygen to produce an alcohol with Markovnikov selectivity.
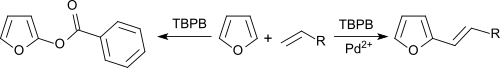
In chemistry, the Halcon process refers to technology for the production of propylene oxide by oxidation of propylene with tert-butyl hydroperoxide. The reaction requires metal catalysts, which typically contain molybdenum: