Related Research Articles

Reuptake is the reabsorption of a neurotransmitter by a neurotransmitter transporter located along the plasma membrane of an axon terminal or glial cell after it has performed its function of transmitting a neural impulse.
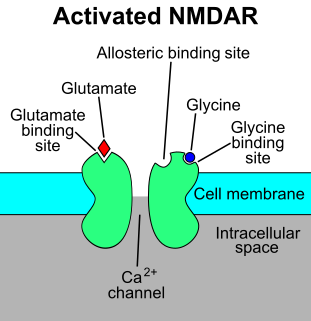
The N-methyl-D-aspartatereceptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and ion channel found in neurons. The NMDA receptor is one of three types of ionotropic glutamate receptors, the other two being AMPA and kainate receptors. Depending on its subunit composition, its ligands are glutamate and glycine (or D-serine). However, the binding of the ligands is typically not sufficient to open the channel as it may be blocked by Mg2+ ions which are only removed when the neuron is sufficiently depolarized. Thus, the channel acts as a “coincidence detector” and only once both of these conditions are met, the channel opens and it allows positively charged ions (cations) to flow through the cell membrane. The NMDA receptor is thought to be very important for controlling synaptic plasticity and mediating learning and memory functions.

Cyclic nucleotide–gated ion channels or CNG channels are ion channels that function in response to the binding of cyclic nucleotides. CNG channels are nonselective cation channels that are found in the membranes of various tissue and cell types, and are significant in sensory transduction as well as cellular development. Their function can be the result of a combination of the binding of cyclic nucleotides and either a depolarization or a hyperpolarization event. Initially discovered in the cells that make up the retina of the eye, CNG channels have been found in many different cell types across both the animal and the plant kingdoms. CNG channels have a very complex structure with various subunits and domains that play a critical role in their function. CNG channels are significant in the function of various sensory pathways including vision and olfaction, as well as in other key cellular functions such as hormone release and chemotaxis. CNG channels have also been found to exist in prokaryotes, including many spirochaeta, though their precise role in bacterial physiology remains unknown.

Ligand-gated ion channels (LICs, LGIC), also commonly referred to as ionotropic receptors, are a group of transmembrane ion-channel proteins which open to allow ions such as Na+, K+, Ca2+, and/or Cl− to pass through the membrane in response to the binding of a chemical messenger (i.e. a ligand), such as a neurotransmitter.
Chromosome 8 is one of the 23 pairs of chromosomes in humans. People normally have two copies of this chromosome. Chromosome 8 spans about 145 million base pairs and represents between 4.5 and 5.0% of the total DNA in cells.
The PDZ domain is a common structural domain of 80-90 amino-acids found in the signaling proteins of bacteria, yeast, plants, viruses and animals. Proteins containing PDZ domains play a key role in anchoring receptor proteins in the membrane to cytoskeletal components. Proteins with these domains help hold together and organize signaling complexes at cellular membranes. These domains play a key role in the formation and function of signal transduction complexes. PDZ domains also play a highly significant role in the anchoring of cell surface receptors to the actin cytoskeleton via mediators like NHERF and ezrin.

CDK-activating kinase (CAK) activates the cyclin-CDK complex by phosphorylating threonine residue 160 in the CDK activation loop. CAK itself is a member of the Cdk family and functions as a positive regulator of Cdk1, Cdk2, Cdk4, and Cdk6.

The EF hand is a helix–loop–helix structural domain or motif found in a large family of calcium-binding proteins.

Mitochondrial carriers are proteins from solute carrier family 25 which transfer molecules across the membranes of the mitochondria. Mitochondrial carriers are also classified in the Transporter Classification Database. The Mitochondrial Carrier (MC) Superfamily has been expanded to include both the original Mitochondrial Carrier (MC) family and the Mitochondrial Inner/Outer Membrane Fusion (MMF) family.

Ifenprodil is an inhibitor of the NMDA receptor, specifically of GluN1 and GluN2B subunits. Additionally, ifenprodil inhibits GIRK channels, and interacts with alpha1 adrenergic, serotonin, and sigma receptors.

Glutamate [NMDA] receptor subunit epsilon-2, also known as N-methyl D-aspartate receptor subtype 2B, is a protein that in humans is encoded by the GRIN2B gene.

Glutamate [NMDA] receptor subunit epsilon-1 is a protein that in humans is encoded by the GRIN2A gene.
Glutamate [NMDA] receptor subunit zeta-1 is a protein that in humans is encoded by the GRIN1 gene.

Glutamate [NMDA] receptor subunit 3A is a protein that in humans is encoded by the GRIN3A gene.

Glutamate [NMDA] receptor subunit 3B is a protein that in humans is encoded by the GRIN3B gene.
The Walker A and Walker B motifs are protein sequence motifs, known to have highly conserved three-dimensional structures. These were first reported in ATP-binding proteins by Walker and co-workers in 1982.
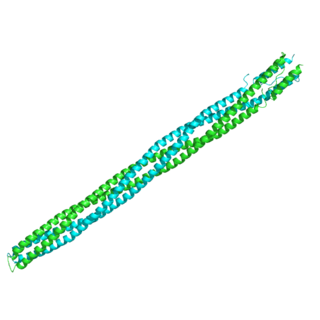
The Methyl-accepting chemotaxis proteins are a family of transmembrane receptors that mediate chemotactic response in certain enteric bacteria, such as Salmonella typhimurium and Escherichia coli. These methyl-accepting chemotaxis receptors are one of the first components in the sensory excitation and adaptation responses in bacteria, which act to alter swimming behaviour upon detection of specific chemicals. Use of the MCP allows bacteria to detect concentrations of molecules in the extracellular matrix so that the bacteria may smooth swim or tumble accordingly. If the bacterium detects rising levels of attractants (nutrients) or declining levels of repellents (toxins), the bacterium will continue swimming forward, or smooth swimming. If the bacterium detects declining levels of attractants or rising levels of repellents, the bacterium will tumble and re-orient itself in a new direction. In this manner, a bacterium may swim towards nutrients and away from toxins
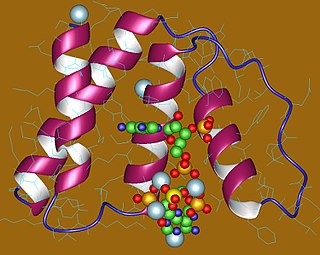
In molecular biology, the Acyl-CoA-binding protein (ACBP) is a small protein that binds medium- and long-chain acyl-CoA esters with very high affinity and may function as an intracellular carrier of acyl-CoA esters. ACBP is also known as diazepam binding inhibitor (DBI) or endozepine (EP) because of its ability to displace diazepam from the benzodiazepine (BZD) recognition site located on the GABA type A receptor. It is therefore possible that this protein also acts as a neuropeptide to modulate the action of the GABA receptor.
Conantokins are a small family of helical peptides that are derived from the venom of predatory marine snails of the genus Conus. Conantokins act as potent and specific antagonists of the N-methyl-D-aspartate receptor (NMDAR). They are the only naturally-derived peptides to do so. The subtypes of conantokins exhibit a surprising variability of selectivity across the NMDAR subunits, and are therefore uniquely useful in developing subunit-specific pharmacological probes.
A FFAT motif is a protein sequence motif of six defined amino acids plus neighbouring residues that binds to proteins in the VAP protein family.
References
- 1 2 van Stelten J, Silva F, Belin D, Silhavy TJ (August 2009). "Effects of antibiotics and a proto-oncogene homolog on destruction of protein translocator SecY". Science. 325 (5941): 753–6. Bibcode:2009Sci...325..753V. doi:10.1126/science.1172221. PMC 2832214 . PMID 19661432.
- 1 2 Bultynck G, Kiviluoto S, Henke N, Ivanova H, Schneider L, Rybalchenko V, Luyten T, Nuyts K, De Borggraeve W, Bezprozvanny I, Parys JB, De Smedt H, Missiaen L, Methner A (January 2012). "The C terminus of Bax inhibitor-1 forms a Ca2+-permeable channel pore". The Journal of Biological Chemistry. 287 (4): 2544–57. doi: 10.1074/jbc.M111.275354 . PMC 3268414 . PMID 22128171.
- 1 2 Chang Y, Bruni R, Kloss B, Assur Z, Kloppmann E, Rost B, Hendrickson WA, Liu Q (June 2014). "Structural basis for a pH-sensitive calcium leak across membranes". Science. 344 (6188): 1131–5. Bibcode:2014Sci...344.1131C. doi:10.1126/science.1252043. PMC 4119810 . PMID 24904158.
As of this edit, this article uses content from "1.A.14 The Testis-Enhanced Gene Transfer (TEGT) Family" , which is licensed in a way that permits reuse under the Creative Commons Attribution-ShareAlike 3.0 Unported License, but not under the GFDL. All relevant terms must be followed.