
5-Methylcytosine is a methylated form of the DNA base cytosine (C) that regulates gene transcription and takes several other biological roles. When cytosine is methylated, the DNA maintains the same sequence, but the expression of methylated genes can be altered. 5-Methylcytosine is incorporated in the nucleoside 5-methylcytidine.
Demethylation is the chemical process resulting in the removal of a methyl group (CH3) from a molecule. A common way of demethylation is the replacement of a methyl group by a hydrogen atom, resulting in a net loss of one carbon and two hydrogen atoms.

The CpG sites or CG sites are regions of DNA where a cytosine nucleotide is followed by a guanine nucleotide in the linear sequence of bases along its 5' → 3' direction. CpG sites occur with high frequency in genomic regions called CpG islands.
In biology, reprogramming refers to erasure and remodeling of epigenetic marks, such as DNA methylation, during mammalian development or in cell culture. Such control is also often associated with alternative covalent modifications of histones.

Acute myeloid leukemia (AML) is a cancer of the myeloid line of blood cells, characterized by the rapid growth of abnormal cells that build up in the bone marrow and blood and interfere with normal blood cell production. Symptoms may include feeling tired, shortness of breath, easy bruising and bleeding, and increased risk of infection. Occasionally, spread may occur to the brain, skin, or gums. As an acute leukemia, AML progresses rapidly, and is typically fatal within weeks or months if left untreated.
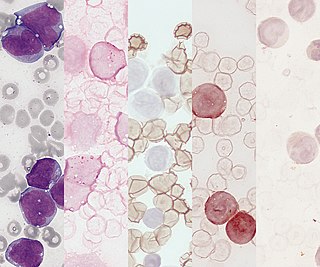
Acute monocytic leukemia is a type of acute myeloid leukemia. In AML-M5 >80% of the leukemic cells are of monocytic lineage. This cancer is characterized by a dominance of monocytes in the bone marrow. There is an overproduction of monocytes that the body does not need in the periphery. These overproduced monocytes interfere with normal immune cell production which causes many health complications for the affected individual.

Acute myeloblastic leukemia with maturation (M2) is a subtype of acute myeloid leukemia (AML).
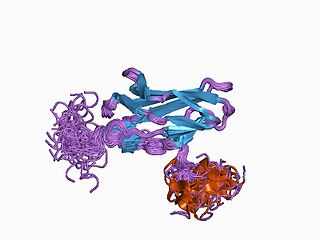
Runt-related transcription factor 1 (RUNX1) also known as acute myeloid leukemia 1 protein (AML1) or core-binding factor subunit alpha-2 (CBFA2) is a protein that in humans is encoded by the RUNX1 gene.

MN1 is a gene found on human chromosome 22, with gene map locus 22q12.3-qter. Its official full name is meningioma 1 because it is disrupted by a balanced translocation (4;22) in a meningioma.

Wilms tumor protein (WT33) is a protein that in humans is encoded by the WT1 gene on chromosome 11p.

Homeobox protein Hox-A9 is a protein that in humans is encoded by the HOXA9 gene.

G/T mismatch-specific thymine DNA glycosylase is an enzyme that in humans is encoded by the TDG gene. Several bacterial proteins have strong sequence homology with this protein.

For molecular biology in mammals, DNA demethylation causes replacement of 5-methylcytosine (5mC) in a DNA sequence by cytosine (C). DNA demethylation can occur by an active process at the site of a 5mC in a DNA sequence or, in replicating cells, by preventing addition of methyl groups to DNA so that the replicated DNA will largely have cytosine in the DNA sequence.

5-Hydroxymethylcytosine (5hmC) is a DNA pyrimidine nitrogen base derived from cytosine. It is potentially important in epigenetics, because the hydroxymethyl group on the cytosine can possibly switch a gene on and off. It was first seen in bacteriophages in 1952. However, in 2009 it was found to be abundant in human and mouse brains, as well as in embryonic stem cells. In mammals, it can be generated by oxidation of 5-methylcytosine, a reaction mediated by TET enzymes. Its molecular formula is C5H7N3O2.

Ten-eleven translocation methylcytosine dioxygenase 1 (TET1) is a member of the TET family of enzymes, in humans it is encoded by the TET1 gene. Its function, regulation, and utilizable pathways remain a matter of current research while it seems to be involved in DNA demethylation and therefore gene regulation.

Tet methylcytosine dioxygenase 3 is a protein that in humans is encoded by the TET3 gene.
Anjana Rao is a cellular and molecular biologist of Indian ethnicity, working in the US. She uses immune cells as well as other types of cells to understand intracellular signaling and gene expression. Her research focuses on how signaling pathways control gene expression.

The TET enzymes are a family of ten-eleven translocation (TET) methylcytosine dioxygenases. They are instrumental in DNA demethylation. 5-Methylcytosine is a methylated form of the DNA base cytosine (C) that often regulates gene transcription and has several other functions in the genome.
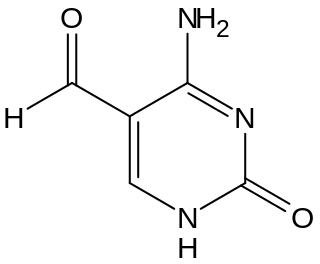
5-Formylcytosine (5fC) is a pyrimidine nitrogen base derived from cytosine. In the context of nucleic acid chemistry and biology, it is regarded as an epigenetic marker. Discovered in 2011 in mammalian embryonic stem cells by Thomas Carell's research group the modified nucleoside was more recently confirmed to be relevant both as an intermediate in the active demethylation pathway and as a standalone epigenetic marker. In mammals, 5fC is formed by oxidation of 5-Hydroxymethylcytosine (5hmC) a reaction mediated by TET enzymes. Its molecular formula is C5H5N3O2.

Beck–Fahrner syndrome, also known as BEFAHRS and TET3 deficiency, is a rare genetic disorder caused by mutations of the TET3 gene. It can occur de novo or can be inherited in an autosomal dominant manner. Mutations in the TET3 gene disrupts DNA demethylation during early embryogenesis and neural development. Most common clinical presentation includes global developmental delay, psychomotor retardation, neurodevelopmental disorders, hypotonia, epilepsy and dysmorphic features. It is diagnosed using molecular and genetic testing in setting of typical symptoms. Management is supportive and intended to improve quality of life.




















