
A crystal or crystalline solid is a solid material whose constituents are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification.

Cubic zirconia (abbreviated CZ) is the cubic crystalline form of zirconium dioxide (ZrO2). The synthesized material is hard and usually colorless, but may be made in a variety of different colors. It should not be confused with zircon, which is a zirconium silicate (ZrSiO4). It is sometimes erroneously called cubic zirconium.

Zirconium dioxide is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant stabilized cubic structured zirconia, cubic zirconia, is synthesized in various colours for use as a gemstone and a diamond simulant.

In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (a by a) and height (c, which is different from a).
Polishing is the process of creating a smooth and shiny surface by rubbing it or by applying a chemical treatment, leaving a clean surface with a significant specular reflection. In some materials, polishing is also able to reduce diffuse reflection to minimal values.
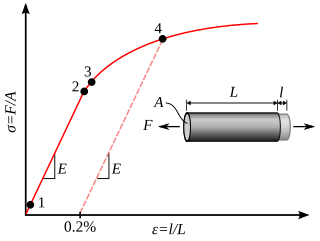
In physics and materials science, plasticity is the ability of a solid material to undergo permanent deformation, a non-reversible change of shape in response to applied forces. For example, a solid piece of metal being bent or pounded into a new shape displays plasticity as permanent changes occur within the material itself. In engineering, the transition from elastic behavior to plastic behavior is known as yielding.
In materials science, superplasticity is a state in which solid crystalline material is deformed well beyond its usual breaking point, usually over about 400% during tensile deformation. Such a state is usually achieved at high homologous temperature. Examples of superplastic materials are some fine-grained metals and ceramics. Other non-crystalline materials (amorphous) such as silica glass and polymers also deform similarly, but are not called superplastic, because they are not crystalline; rather, their deformation is often described as Newtonian fluid. Superplastically deformed material gets thinner in a very uniform manner, rather than forming a "neck" that leads to fracture. Also, the formation of microvoids, which is another cause of early fracture, is inhibited. Superplasticity must not be confused with superelasticity.

In dentistry, a crown or a dental cap is a type of dental restoration that completely caps or encircles a tooth or dental implant. A crown may be needed when a large dental cavity threatens the health of a tooth. A crown is typically bonded to the tooth by dental cement. They can be made from various materials, which are usually fabricated using indirect methods. Crowns are used to improve the strength or appearance of teeth and to halt deterioration. While beneficial to dental health, the procedure and materials can be costly.

Bismuth(III) oxide is perhaps the most industrially important compound of bismuth. It is also a common starting point for bismuth chemistry. It is found naturally as the mineral bismite (monoclinic) and sphaerobismoite, but it is usually obtained as a by-product of the smelting of copper and lead ores. Dibismuth trioxide is commonly used to produce the "Dragon's eggs" effect in fireworks, as a replacement of red lead.

A ceramic knife is a knife with a ceramic blade typically made from zirconium dioxide (ZrO2; also known as zirconia), rather than the steel used for most knives. Ceramic knife blades are usually produced through the dry-pressing and firing of powdered zirconia using solid-state sintering. The blades typically score 8.5 on the Mohs scale of mineral hardness, compared to 4.5 for normal steel and 7.5 to 8 for hardened steel and 10 for diamond. The resultant blade has a hard edge that stays sharp for much longer than conventional steel blades. However, the blade is brittle, subject to chipping, and will break rather than flex if twisted. The ceramic blade is sharpened by grinding the edges with a diamond-dust-coated grinding wheel.

Hafnium(IV) oxide is the inorganic compound with the formula HfO
2. Also known as hafnium dioxide or hafnia, this colourless solid is one of the most common and stable compounds of hafnium. It is an electrical insulator with a band gap of 5.3~5.7 eV. Hafnium dioxide is an intermediate in some processes that give hafnium metal.
Pseudoelasticity, sometimes called superelasticity, is an elastic (reversible) response to an applied stress, caused by a phase transformation between the austenitic and martensitic phases of a crystal. It is exhibited in shape-memory alloys.
Zirconia toughened alumina is a ceramic material comprising alumina and zirconia. It is a composite ceramic material with zirconia grains in the alumina matrix.

Yttria-stabilized zirconia (YSZ) is a ceramic in which the cubic crystal structure of zirconium dioxide is made stable at room temperature by an addition of yttrium oxide. These oxides are commonly called "zirconia" (ZrO2) and "yttria" (Y2O3), hence the name.
A resin-retained bridge is a bridge replacing a missing tooth that relies for its retention on a composite resin cement. It is one of many available dental restoration methods which is considered minimally invasive and conservative of tooth tissue. The resin-retained-bridge has gone through a number of iterations. Perhaps the best known is the Maryland bridge and other designs used in the past include the Rochette bridge. The five-year survival rate is around 83.6% and the ten-year rate at 64.9%. The case selection is important and as with any dental prosthesis, good oral hygiene is paramount for success. In recent years, the indications for the use of resin-retained-bridges have diminished significantly and there have been changes in the principles underpinning their design. Resin-retained-bridges should be considered when a fixed prosthesis retained by natural teeth is required. The use has been driven by the advent of evidence-based dentistry showing the benefits to patients of reduced tooth preparation and the importance of an intact enamel structure for the long-term health of the teeth. The bridge is currently in favour in the United Kingdom for these reasons. Indeed, recent contemporary research shows resin retained bridges have better success rates than implants and are a cheaper alternative.

Ulimorelin is a drug with a modified cyclic peptide structure which acts as a selective agonist of the ghrelin/growth hormone secretagogue receptor (GHSR-1a).. Unlike many related drugs, ulimorelin has little or no effect on growth hormone (GH) release in rats. However, like ghrelin and other ghrelin agonists, ulimorelin does stimulate GH release with concomitant increases in insulin-like growth factor 1 (IGF-1) in humans. It has been researched for enhancing gastrointestinal motility, especially in gastroparesis and in aiding recovery of bowel function following gastrointestinal surgery, where opioid analgesic drugs used for post-operative pain relief may worsen existing constipation. While ulimorelin has been shown to increase both upper and lower gastrointestinal motility in rats, and showed promising results initially in humans, it failed in pivotal clinical trials in post operative ileus.
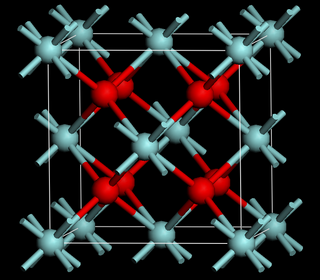
Ceria-zirconia is a solid solution of cerium(IV) oxide (CeO2, also known as ceria) and zirconium oxide (ZrO2, also known as zirconia).
10-Methacryloyloxydecyl dihydrogen phosphate is used for dental adhesive materials. The phosphate monomer was developed by Kuraray co., Ltd. with focus on the dental adhesion technology in 1981.
Anil Vasudeo Virkar is an American materials scientist and engineer, currently Distinguished Professor at University of Utah. He is a Fellow of the ASM International.

Geо́rge Antо́novych Gogо́tsi is a soviet Ukrainian scientist, professor of solid mechanics, doctor of science, and leading researcher of the Pisarenko Institute for Problems of Strength of the National Academy of Sciences of Ukraine.












