
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.

In organic chemistry, a sulfide or thioether is an organosulfur functional group with the connectivity R−S−R' as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application.
A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is formed between one hybridized orbital from each of the carbon atoms. In ethane, the orbitals are sp3-hybridized orbitals, but single bonds formed between carbon atoms with other hybridizations do occur. In fact, the carbon atoms in the single bond need not be of the same hybridization. Carbon atoms can also form double bonds in compounds called alkenes or triple bonds in compounds called alkynes. A double bond is formed with an sp2-hybridized orbital and a p-orbital that is not involved in the hybridization. A triple bond is formed with an sp-hybridized orbital and two p-orbitals from each atom. The use of the p-orbitals forms a pi bond.
In organic chemistry, hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention: production capacity reached 6.6×106 tons in 1995. It is important because aldehydes are easily converted into many secondary products. For example, the resultant aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in speciality chemicals, relevant to the organic synthesis of fragrances and pharmaceuticals. The development of hydroformylation is one of the premier achievements of 20th-century industrial chemistry.

Sulfur monoxide is an inorganic compound with formula SO. It is only found as a dilute gas phase. When concentrated or condensed, it converts to S2O2 (disulfur dioxide). It has been detected in space but is rarely encountered intact otherwise.
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly found as counterions for cationic metal complexes with an unsaturated coordination sphere. These special anions are essential components of homogeneous alkene polymerisation catalysts, where the active catalyst is a coordinatively unsaturated, cationic transition metal complex. For example, they are employed as counterions for the 14 valence electron cations [(C5H5)2ZrR]+ (R = methyl or a growing polyethylene chain). Complexes derived from non-coordinating anions have been used to catalyze hydrogenation, hydrosilylation, oligomerization, and the living polymerization of alkenes. The popularization of non-coordinating anions has contributed to increased understanding of agostic complexes wherein hydrocarbons and hydrogen serve as ligands. Non-coordinating anions are important components of many superacids, which result from the combination of Brønsted acids and Lewis acids.
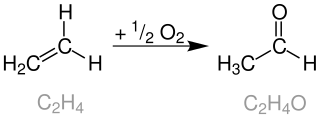
The Wacker process or the Hoechst-Wacker process refers to the oxidation of ethylene to acetaldehyde in the presence of palladium(II) chloride and copper(II) chloride as the catalyst. This chemical reaction was one of the first homogeneous catalysis with organopalladium chemistry applied on an industrial scale.
A transition metal carbene complex is an organometallic compound featuring a divalent carbon ligand, itself also called a carbene. Carbene complexes have been synthesized from most transition metals and f-block metals, using many different synthetic routes such as nucleophilic addition and alpha-hydrogen abstraction. The term carbene ligand is a formalism since many are not directly derived from carbenes and most are much less reactive than lone carbenes. Described often as =CR2, carbene ligands are intermediate between alkyls (−CR3) and carbynes (≡CR). Many different carbene-based reagents such as Tebbe's reagent are used in synthesis. They also feature in catalytic reactions, especially alkene metathesis, and are of value in both industrial heterogeneous and in homogeneous catalysis for laboratory- and industrial-scale preparation of fine chemicals.

In organic chemistry an enol ether is an alkene with an alkoxy substituent. The general structure is R2C=CR-OR where R = H, alkyl or aryl. A common subfamily of enol ethers are vinyl ethers, with the formula ROCH=CH2. Important enol ethers include the reagent 3,4-dihydropyran and the monomers methyl vinyl ether and ethyl vinyl ether.
Endohedral hydrogen fullerene (H2@C60) is an endohedral fullerene containing molecular hydrogen. This chemical compound has a potential application in molecular electronics and was synthesized in 2005 at Kyoto University by the group of Koichi Komatsu. Ordinarily the payload of endohedral fullerenes are inserted at the time of the synthesis of the fullerene itself or is introduced to the fullerene at very low yields at high temperatures and high pressure. This particular fullerene was synthesised in an unusual way in three steps starting from pristine C60 fullerene: cracking open the carbon framework, insert hydrogen gas and zipping up by organic synthesis methods.
![<span class="mw-page-title-main">Eschenmoser's salt</span> Ionic compound with the formula [(H3C–)2N–CH2]I](https://upload.wikimedia.org/wikipedia/commons/thumb/2/28/Eschenmosersalz.png/320px-Eschenmosersalz.png)
In organic chemistry, Eschenmoser's salt is the ionic, organic compound [(CH3)2NCH2]I. It is the iodide salt of the dimethylaminomethylene cation [(CH3)2NCH2]+.
Asymmetric hydrogenation is a chemical reaction that adds two atoms of hydrogen to a target (substrate) molecule with three-dimensional spatial selectivity. Critically, this selectivity does not come from the target molecule itself, but from other reagents or catalysts present in the reaction. This allows spatial information to transfer from one molecule to the target, forming the product as a single enantiomer. The chiral information is most commonly contained in a catalyst and, in this case, the information in a single molecule of catalyst may be transferred to many substrate molecules, amplifying the amount of chiral information present. Similar processes occur in nature, where a chiral molecule like an enzyme can catalyse the introduction of a chiral centre to give a product as a single enantiomer, such as amino acids, that a cell needs to function. By imitating this process, chemists can generate many novel synthetic molecules that interact with biological systems in specific ways, leading to new pharmaceutical agents and agrochemicals. The importance of asymmetric hydrogenation in both academia and industry contributed to two of its pioneers — William Standish Knowles and Ryōji Noyori — being collectively awarded one half of the 2001 Nobel Prize in Chemistry.
Organoplatinum chemistry is the chemistry of organometallic compounds containing a carbon to platinum chemical bond, and the study of platinum as a catalyst in organic reactions. Organoplatinum compounds exist in oxidation state 0 to IV, with oxidation state II most abundant. The general order in bond strength is Pt-C (sp) > Pt-O > Pt-N > Pt-C (sp3). Organoplatinum and organopalladium chemistry are similar, but organoplatinum compounds are more stable and therefore less useful as catalysts.

Brookhart's acid is the salt of the diethyl ether oxonium ion and tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (BAr′4). It is a colorless solid, used as a strong acid. The compound was first reported by Volpe, Grant, and Brookhart in 1992.

Tetramethylurea is the organic compound with the formula (Me2N)2CO. It is a substituted urea. This colorless liquid is used as an aprotic-polar solvent, especially for aromatic compounds and is used e. g. for Grignard reagents.

Tris(dimethylamino)methane (TDAM) is the simplest representative of the tris(dialkylamino)methanes of the general formula (R2N)3CH in which three of the four of methane's hydrogen atoms are replaced by dimethylamino groups (−N(CH3)2). Tris(dimethylamino)methane can be regarded as both an amine and an orthoamide.

Organotantalum chemistry is the chemistry of chemical compounds containing a carbon-to-tantalum chemical bond. A wide variety of compound have been reported, initially with cyclopentadienyl and CO ligands. Oxidation states vary from Ta(V) to Ta(-I).
In organic chemistry, Wittig reagents are organophosphorus compounds of the formula R3P=CHR', where R is usually phenyl. They are used to convert ketones and aldehydes to alkenes:

trans,trans,trans-(1,5,9 Cyclododecatriene)nickel(0) a organonickel compound with the formula NiC12H18, better known as t-Ni(cdt). It is a 16-electron coordination complex featuring trigonal planar nickel (0) bound to the three alkene groups in the cyclododecatriene ligand. X-ray structural analysis demonstrates that the three olefins adopt a propeller-like arrangement around the nickel atom center, making the structure chiral. This extremely air-sensitive deep red solid was the first discovered Ni(0)-olefin complex.
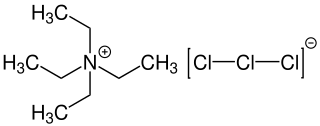
Tetraethylammonium trichloride (also known as Mioskowski reagent) is a chemical compound with the formula [NEt4][Cl3] consisting of a tetraethylammonium cation and a trichloride as anion. The trichloride is also known as trichlorine monoanion representing one of the simplest polychlorine anions. Tetraethylammonium trichloride is used as reagent for chlorinations and oxidation reactions.












![<span class="mw-page-title-main">Eschenmoser's salt</span> Ionic compound with the formula [(H3C–)2N–CH2]I](https://upload.wikimedia.org/wikipedia/commons/thumb/2/28/Eschenmosersalz.png/320px-Eschenmosersalz.png)





