Ranbaxy Laboratories Limited was an Indian multinational pharmaceutical company that was incorporated in India in 1961 and remained an entity until 2014. The company went public in 1973. Ownership of Ranbaxy changed twice over the course of its history.
Serono was a biotechnology company headquartered in Geneva, Switzerland. It was acquired by the German pharmaceutical company Merck in 2006. The company was founded as the Serono Pharmacological Institute by Cesare Serono in 1906 in Rome, Italy. A key step in its development was the discovery of a method of extracting urinary gonadotropins by Dr. Piero Donini. Serono was incorporated in 1987 and the holding company, Ares-Serono S.A., changed its name to Serono S.A. in May 2000.

Barr Pharmaceuticals was a global specialty and generic drug manufacturer with operations in 30 countries.
Dr. Reddy's Laboratories is an Indian multinational pharmaceutical company based in Hyderabad. The company was founded by Kallam Anji Reddy, who previously worked in the mentor institute Indian Drugs and Pharmaceuticals Limited. Dr. Reddy manufactures and markets a wide range of pharmaceuticals in India and overseas. The company produces over 190 medications, 60 active pharmaceutical ingredients (APIs) for drug manufacture, diagnostic kits, critical care, and biotechnology.

Biocon Limited is an Indian biopharmaceutical company based in Bangalore. It was founded by Kiran Mazumdar-Shaw in 1978. The company manufactures generic active pharmaceutical ingredients (APIs) that are sold in approximately 120 countries, including the United States and Europe. It also manufactures novel biologics as well as biosimilar insulins and antibodies, which are sold in India as branded formulations. Biocon's biosimilar products are also sold in both bulk and formulation forms in several emerging markets.
Mylan N.V. was a global generic and specialty pharmaceuticals company. In November 2020, Mylan merged with Upjohn, Pfizer's off-patent medicine division, to form Viatris. Previously, the company was domiciled in the Netherlands, with principal executive offices in Hatfield, Hertfordshire, UK and a "Global Center" in Canonsburg, Pennsylvania, US.

Pliva d.o.o. is a pharmaceutical company based in Zagreb, Croatia that primarily manufactures and sells generic drugs. It is a subsidiary of Teva Pharmaceuticals.
Sigma-Aldrich is an American chemical, life science, and biotechnology company owned by the multinational chemical conglomerate Merck Group.
The pharmaceutical industry in India was valued at an estimated US$42 billion in 2021 and is estimated to reach $130 billion by 2030. India is the world's largest provider of generic medicines by volume, with a 20% share of total global pharmaceutical exports. It is also the largest vaccine supplier in the world by volume, accounting for more than 60% of all vaccines manufactured in the world. Indian pharmaceutical products are exported to various regulated markets including the US, UK, European Union and Canada.
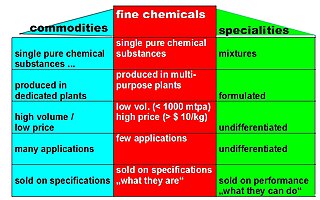
In chemistry, fine chemicals are complex, single, pure chemical substances, produced in limited quantities in multipurpose plants by multistep batch chemical or biotechnological processes. They are described by exacting specifications, used for further processing within the chemical industry and sold for more than $10/kg. The class of fine chemicals is subdivided either on the basis of the added value, or the type of business transaction, namely standard or exclusive products.

Teva Pharmaceutical Industries Ltd. is an Israeli multinational pharmaceutical company. Teva specializes primarily in generic drugs, but other business interests include branded-drugs, active pharmaceutical ingredients (API's) and, to a lesser extent, contract manufacturing services and an out-licensing platform.

Actavis Generics is a global pharmaceutical company focused on acquiring, developing, manufacturing and marketing branded pharmaceuticals, generic and over-the-counter medicines, and biologic products. Actavis has a commercial presence across approximately 100 countries. The company has global headquarters in Dublin, Ireland and administrative headquarters in Parsippany-Troy Hills, New Jersey, United States.

Cambrex Corporation is a Contract Development Manufacturing Organisation (CDMO) that provides drug substance, drug product and analytical services across the entire drug lifecycle, as well as active pharmaceutical ingredients (APIs). With over 2,200 employees in 7 countries across 13 locations, Cambrex operates in branded and generic markets for API and dosage form development and manufacturing.

Teva Canada is one of Canada's largest generic pharmaceutical companies. The company was founded as Novopharm by Leslie Dan in 1965. After its acquisition by pharmaceutical giant Teva Pharmaceutical Industries in 2000, it was renamed Teva Novopharm. The Novopharm name was dropped in 2010, when it became Teva Canada.

Sun Pharmaceutical Industries Limited is an Indian multinational pharmaceutical company headquartered in Mumbai, that manufactures and sells pharmaceutical formulations and active pharmaceutical ingredients (APIs) in more than 100 countries across the globe. It is the largest pharmaceutical company in India and the fourth largest specialty generic pharmaceutical company in the world. The products cater to a vast range of therapeutic segments covering psychiatry, anti-infectives, neurology, cardiology, diabetology, gastroenterology, ophthalmology, nephrology, urology, dermatology, gynecology, respiratory, oncology, dental and nutritionals.
Hovione is a Contract Development and Manufacturing Organization (CDMO) with services for drug substance, drug product intermediate and drug product. The company has four FDA inspected sites in the United States, Portugal, Ireland and China development laboratories in Lisbon, Portugal and New Jersey, USA. Hovione is also present in the inhalation area, and provides a complete range of services, from active pharmaceutical ingredients (APIs), formulation development and devices. Hovione was the first Chemical/ Pharmaceutical Company to become a Certified B Corporation (certification), is a member of Rx-360 and EFCG.
Dishman Carbogen Amcis Ltd is an Indian multinational pharmaceutical company specialized in the manufacture of active ingredients and contract development and manufacturing. Dishman employs over 1,000 people worldwide and is listed on the Bombay Stock Exchange (BSE) and National Stock Exchange of India (NSE).
Allergan plc is an American, Irish-domiciled pharmaceutical company that acquires, develops, manufactures and markets brand name drugs and medical devices in the areas of medical aesthetics, eye care, central nervous system, and gastroenterology. The company is the maker of Botox.
Alembic Pharmaceuticals Ltd. is an Indian multinational pharmaceutical company headquartered in Vadodara. It is involved in the manufacture of pharmaceutical products, pharmaceutical substances and intermediates. It is also termed to be a market leader in macrolides segment of anti-infective drugs in India.

Theramex is a pharmaceutical company based in London which produces women's health products focusing on contraception, fertility, menopause and osteoporosis. It was established in 2018 with the acquisition of some of the assets of Teva Active Pharmaceutical Ingredients relating to Theramex Laboratories, a pharmaceutical company which was based in Monaco. Brands include Ovaleap, Zoely, Seasonique, Actonel, Estreva and Lutenyl. It made an agreement with TherapeuticsMD in 2019 for exclusive licensing and supply rights to Bijuva and Imvexxy outside of the USA, Canada and Israel for which it paid a license fee of $15.5 million. In 2021, Theramex launched Livogiva, Lundeos (Osteoporosis) and Bijuva (Menopause). That same year Theramex entered Consumer Healthcare Market with Femarelle, a non-hormonal option to treat menopause symptoms.









