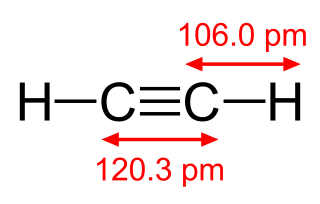
Acetylene is the chemical compound with the formula C2H2 and structure H−C≡C−H. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine.

A Bunsen burner, named after Robert Bunsen, is a kind of ambient air gas burner used as laboratory equipment; it produces a single open gas flame, and is used for heating, sterilization, and combustion.

Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining.

Carbon monoxide is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest carbon oxide. In coordination complexes, the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry.

In thermodynamics, an exothermic process is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light, electricity, or sound. The term exothermic was first coined by 19th-century French chemist Marcellin Berthelot.

Fire is the rapid oxidation of a material in the exothermic chemical process of combustion, releasing heat, light, and various reaction products. At a certain point in the combustion reaction, called the ignition point, flames are produced. The flame is the visible portion of the fire. Flames consist primarily of carbon dioxide, water vapor, oxygen and nitrogen. If hot enough, the gases may become ionized to produce plasma. Depending on the substances alight, and any impurities outside, the color of the flame and the fire's intensity will be different.

Hydrogen is a chemical element; it has symbol H and atomic number 1. It is the lightest element and, at standard conditions, is a gas of diatomic molecules with the formula H2. It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen each atom has one proton, one electron, and no neutrons.

Michael Faraday was an English scientist who contributed to the study of electromagnetism and electrochemistry. His main discoveries include the principles underlying electromagnetic induction, diamagnetism and electrolysis. Although Faraday received little formal education, as a self-made man, he was one of the most influential scientists in history. It was by his research on the magnetic field around a conductor carrying a direct current that Faraday established the concept of the electromagnetic field in physics. Faraday also established that magnetism could affect rays of light and that there was an underlying relationship between the two phenomena. He similarly discovered the principles of electromagnetic induction, diamagnetism, and the laws of electrolysis. His inventions of electromagnetic rotary devices formed the foundation of electric motor technology, and it was largely due to his efforts that electricity became practical for use in technology.

Oxygen is a chemical element; it has symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is the most abundant element in Earth's crust, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula O
2. Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity".

Ethane is a naturally occurring organic chemical compound with chemical formula C
2H
6. At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining. Its chief use is as feedstock for ethylene production.

A flame is the visible, gaseous part of a fire. It is caused by a highly exothermic chemical reaction taking place in a thin zone. When flames are hot enough to have ionized gaseous components of sufficient density, they are then considered plasma.

Jean Baptiste André Dumas was a French chemist, best known for his works on organic analysis and synthesis, as well as the determination of atomic weights and molecular weights by measuring vapor densities. He also developed a method for the analysis of nitrogen in compounds.
The heating value of a substance, usually a fuel or food, is the amount of heat released during the combustion of a specified amount of it.
A direct combination reaction (also known as a synthesis reaction) is a reaction where two or more elements or compounds (reactants) combine to form a single compound (product). Such reactions are represented by equations of the following form: X + Y → XY (A+B → AB). The combination of two or more elements to form one compound is called a combination reaction. In other words, when two or more elements or compounds react so as to form one single compound, then the chemical reaction that takes place is called a combination reaction. | a)- Between elements | C + O2 → CO2 | Carbon completely burnt in oxygen yields carbon dioxide |- | b) Between compounds | CaO + H2O → Ca(OH)2 | Calcium oxide (lime) combined with water gives calcium hydroxide (slaked lime) |- | c) Between elements and compounds | 2CO + O2 → 2CO2 | Oxygen combines with carbon monoxide,And carbon dioxide is formed. |}

Industrial gases are the gaseous materials that are manufactured for use in industry. The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene, although many other gases and mixtures are also available in gas cylinders. The industry producing these gases is also known as industrial gas, which is seen as also encompassing the supply of equipment and technology to produce and use the gases. Their production is a part of the wider chemical Industry.

A Hofmann voltameter is an apparatus for electrolysing water, invented by August Wilhelm von Hofmann (1818–1892) in 1866. It consists of three joined upright cylinders, usually glass. The inner cylinder is open at the top to allow addition of water which contains a low concentration of a compound such as sulfuric acid to improve conductivity and complete the circuit. A platinum electrode is placed inside the bottom of each of the two side cylinders, connected to the positive and negative terminals of a source of electricity. When current is run through Hofmann's voltameter, gaseous oxygen forms at the anode and gaseous hydrogen at the cathode. Each gas displaces water and collects at the top of the two outer tubes.

In chemistry, the CPK coloring is a popular color convention for distinguishing atoms of different chemical elements in molecular models.

A luminous flame is a burning flame which is brightly visible. Much of its output is in the form of visible light, as well as heat or light in the non-visible wavelengths.

A splint is a simple piece of equipment used in scientific laboratories. Splints are typically long, thin strips of wood, about 6 inches (15 cm) long and ¼ inch (6 mm) wide, and are consumable but inexpensive. They are typically used for tasks such as lighting bunsen burners, as the length of the splint allows a flame to be lit without risk to the user's hand, should the burner flare back. Another use for splints are chemical identification of various gases, and splints are also used to teach simple chemical principles in schools and homes.



















