
Nucleotides are organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver.

Thiamine, also known as thiamin and vitamin B1, is a vitamin, an essential micronutrient for humans and animals. It is found in food and commercially synthesized to be a dietary supplement or medication. Phosphorylated forms of thiamine are required for some metabolic reactions, including the breakdown of glucose and amino acids.

In chemistry, pyrophosphates are phosphorus oxyanions that contain two phosphorus atoms in a P−O−P linkage. A number of pyrophosphate salts exist, such as disodium pyrophosphate and tetrasodium pyrophosphate, among others. Often pyrophosphates are called diphosphates. The parent pyrophosphates are derived from partial or complete neutralization of pyrophosphoric acid. The pyrophosphate bond is also sometimes referred to as a phosphoanhydride bond, a naming convention which emphasizes the loss of water that occurs when two phosphates form a new P−O−P bond, and which mirrors the nomenclature for anhydrides of carboxylic acids. Pyrophosphates are found in ATP and other nucleotide triphosphates, which are important in biochemistry. The term pyrophosphate is also the name of esters formed by the condensation of a phosphorylated biological compound with inorganic phosphate, as for dimethylallyl pyrophosphate. This bond is also referred to as a high-energy phosphate bond.
In organic chemistry, a tetrose is a monosaccharide with 4 carbon atoms. They have either an aldehyde functional group in position 1 (aldotetroses) or a ketone group in position 2 (ketotetroses).
A nucleoside triphosphate is a nucleoside containing a nitrogenous base bound to a 5-carbon sugar, with three phosphate groups bound to the sugar. They are the molecular precursors of both DNA and RNA, which are chains of nucleotides made through the processes of DNA replication and transcription. Nucleoside triphosphates also serve as a source of energy for cellular reactions and are involved in signalling pathways.

Transketolase is an enzyme that, in humans, is encoded by the TKT gene. It participates in both the pentose phosphate pathway in all organisms and the Calvin cycle of photosynthesis. Transketolase catalyzes two important reactions, which operate in opposite directions in these two pathways. In the first reaction of the non-oxidative pentose phosphate pathway, the cofactor thiamine diphosphate accepts a 2-carbon fragment from a 5-carbon ketose (D-xylulose-5-P), then transfers this fragment to a 5-carbon aldose (D-ribose-5-P) to form a 7-carbon ketose (sedoheptulose-7-P). The abstraction of two carbons from D-xylulose-5-P yields the 3-carbon aldose glyceraldehyde-3-P. In the Calvin cycle, transketolase catalyzes the reverse reaction, the conversion of sedoheptulose-7-P and glyceraldehyde-3-P to pentoses, the aldose D-ribose-5-P and the ketose D-xylulose-5-P.
Purine nucleoside phosphorylase, PNP, PNPase or inosine phosphorylase is an enzyme that in humans is encoded by the NP gene. It catalyzes the chemical reaction
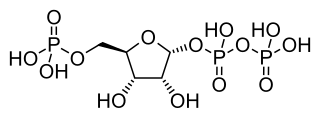
Phosphoribosyl pyrophosphate (PRPP) is a pentose phosphate. It is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, as well as in pyrimidine nucleotide formation. Hence it is a building block for DNA and RNA. The vitamins thiamine and cobalamin, and the amino acid tryptophan also contain fragments derived from PRPP. It is formed from ribose 5-phosphate (R5P) by the enzyme ribose-phosphate diphosphokinase:

Guanosine monophosphate synthetase, also known as GMPS is an enzyme that converts xanthosine monophosphate to guanosine monophosphate.

dCMP deaminase is an enzyme which converts deoxycytidylic acid to deoxyuridylic acid.

The enzyme Inositol phosphate-phosphatase is of the phosphodiesterase family of enzymes. It is involved in the phosphophatidylinositol signaling pathway, which affects a wide array of cell functions, including but not limited to, cell growth, apoptosis, secretion, and information processing. Inhibition of inositol monophosphatase may be key in the action of lithium in treating bipolar disorder, specifically manic depression.

Adenosine 3',5'-bisphosphate is a form of an adenosine nucleotide with two phosphate groups attached to different carbons in the ribose ring. This is distinct from adenosine diphosphate, where the two phosphate groups are attached in a chain to the 5' carbon atom in the ring.
In enzymology, a thiamine-phosphate diphosphorylase is an enzyme that catalyzes the chemical reaction
In enzymology, a nucleoside-phosphate kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a thiamine-diphosphate kinase is an enzyme involved in thiamine metabolism. It catalyzes the chemical reaction
In enzymology, a thiamine kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a thiamine-phosphate kinase is an enzyme that catalyzes the chemical reaction

Inosine-5′-monophosphate dehydrogenase (IMPDH) is a purine biosynthetic enzyme that catalyzes the nicotinamide adenine dinucleotide (NAD+)-dependent oxidation of inosine monophosphate (IMP) to xanthosine monophosphate (XMP), the first committed and rate-limiting step towards the de novo biosynthesis of guanine nucleotides from IMP. IMPDH is a regulator of the intracellular guanine nucleotide pool, and is therefore important for DNA and RNA synthesis, signal transduction, energy transfer, glycoprotein synthesis, as well as other process that are involved in cellular proliferation.
Thymidylate kinase catalyzes the phosphorylation of thymidine 5'-monophosphate (dTMP) to form thymidine 5'-diphosphate (dTDP) in the presence of ATP and magnesium:
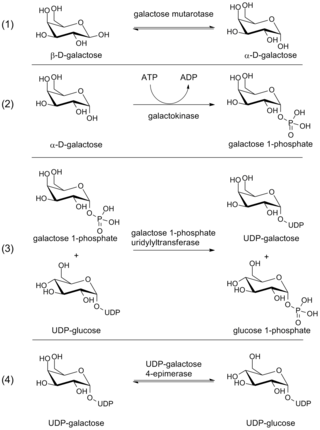
The Leloir pathway is a metabolic pathway for the catabolism of D-galactose. It is named after Luis Federico Leloir, who first described it.













