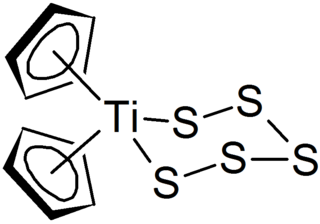The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive elements polonium (Po) and livermorium (Lv). Often, oxygen is treated separately from the other chalcogens, sometimes even excluded from the scope of the term "chalcogen" altogether, due to its very different chemical behavior from sulfur, selenium, tellurium, and polonium. The word "chalcogen" is derived from a combination of the Greek word khalkόs (χαλκός) principally meaning copper, and the Latinized Greek word genēs, meaning born or produced.

In organic chemistry, a thiol, or thiol derivative, is any organosulfur compound of the form R−SH, where R represents an alkyl or other organic substituent. The −SH functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols, and the word is a blend of "thio-" with "alcohol".

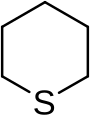
In organic chemistry, a sulfide or thioether is an organosulfur functional group with the connectivity R−S−R' as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application.
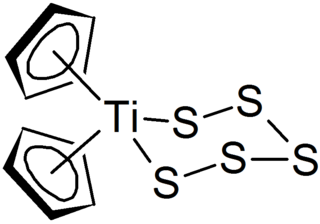
Polysulfides are a class of chemical compounds derived from anionic chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. The inorganic polysulfides have the general formula S2−
n. These anions are the conjugate bases of polysulfanes H2Sn. Organic polysulfides generally have the formulae R1SnR2, where R is an alkyl or aryl group.
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionation, such as when a compound in an intermediate oxidation state is formed from precursors of lower and higher oxidation states, is called comproportionation, also known as symproportionation.
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.

In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and dimethyl sulfoxide (DMSO), a common solvent.

A chalcogenide is a chemical compound consisting of at least one chalcogen anion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for sulfides, selenides, tellurides, and polonides, rather than oxides. Many metal ores exist as chalcogenides. Photoconductive chalcogenide glasses are used in xerography. Some pigments and catalysts are also based on chalcogenides. The metal dichalcogenide MoS2 is a common solid lubricant.

Copper monosulfide is a chemical compound of copper and sulfur. It was initially thought to occur in nature as the dark indigo blue mineral covellite. However, it was later shown to be rather a cuprous compound, formula Cu3S(S2). CuS is a moderate conductor of electricity. A black colloidal precipitate of CuS is formed when hydrogen sulfide, H2S, is bubbled through solutions of Cu(II) salts. It is one of a number of binary compounds of copper and sulfur (see copper sulfide for an overview of this subject), and has attracted interest because of its potential uses in catalysis and photovoltaics.

Phosphorus sulfides comprise a family of inorganic compounds containing only phosphorus and sulfur. These compounds have the formula P4Sn with n ≤ 10. Two are of commercial significance, phosphorus pentasulfide, which is made on a kiloton scale for the production of other organosulfur compounds, and phosphorus sesquisulfide, used in the production of "strike anywhere matches".

Disulfur dichloride is the inorganic compound of sulfur and chlorine with the formula S2Cl2. It is an amber oily liquid.
Selenium hexasulfide is a chemical compound with the chemical formula Se2S6. Its molecular structure is an 8-membered ring, consisting of two selenium and six sulfur atoms (diselenacyclooctasulfane), analogous to the S8 ring, an allotrope of sulfur, and other 8-membered rings of selenium sulfides with formula SenS8−n.

Bismuth(III) sulfide is a chemical compound of bismuth and sulfur. It occurs in nature as the mineral bismuthinite.

Copper(I) sulfide is a copper sulfide, a chemical compound of copper and sulfur. It has the chemical compound Cu2S. It is found in nature as the mineral chalcocite. It has a narrow range of stoichiometry ranging from Cu1.997S to Cu2.000S. Samples are typically black.
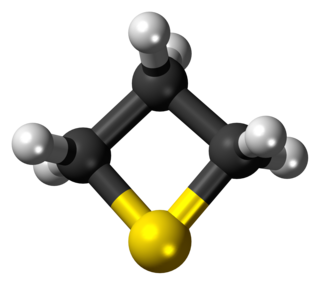
Thietane is a heterocyclic compound containing a saturated four-membered ring with three carbon atoms and one sulfur atom.

Chloro(dimethyl sulfide)gold(I) is a coordination complex of gold. It is a white solid. This compound is a common entry point into gold chemistry.
Hydrogen chalcogenides are binary compounds of hydrogen with chalcogen atoms. Water, the first chemical compound in this series, contains one oxygen atom and two hydrogen atoms, and is the most common compound on the Earth's surface.
Divinyl sulfide is the organosulfur compound with the formula S(CH=CH2)2. A colorless liquid with a faint odor, it is found in some species of Allium.
In organic chemistry, the high dilution principle is a strategy for some macrocyclization reactions, i.e. the synthesis of macrocycles. Unlike the synthesis of 5- and 6-membered rings, the preparation of larger rings competes unfavorably with polymerization reactions. Polymers arise from coupling of long chain precursors. Such reactions are disfavored when the acyclic compounds are dilute. Although high dilution reactions can be conducted in a batch reactor with large volumes of solvent, an alternative practical implementation entails slow addition of reactants, under conditions that the reactants are more rapidly consumed than the rate of addition. Typically, additions use one or more syringe pumps. Illustrative is the synthesis of thiacyclopentadecane from 1,14-dibromotetradecane and sodium sulfide in 45% yield:
Neodymium(III) sulfide is a inorganic chemical compound with the formula Nd2S3 composed of a two neodymium atoms in the +3 oxidation state and three sulfur atoms in the -2 oxidation state. Like other rare earth sulfides, neodymium(III) sulfide is used as a high-performance inorganic pigment.