In chemistry, azide is a linear, polyatomic anion with the formula N−3 and structure −N=N+=N−. It is the conjugate base of hydrazoic acid HN3. Organic azides are organic compounds with the formula RN3, containing the azide functional group. The dominant application of azides is as a propellant in air bags.
In chemistry, halogenation is a chemical reaction which introduces of one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.
Chlorine trifluoride is an interhalogen compound with the formula ClF3. It is a colorless, poisonous, corrosive, and extremely reactive gas that condenses to a pale-greenish yellow liquid, the form in which it is most often sold. It is famous for its extreme oxidation properties. The compound is primarily of interest in plasmaless cleaning and etching operations in the semiconductor industry, in nuclear reactor fuel processing, historically as a component in rocket fuels, and various other industrial operations owing to its corrosive nature.
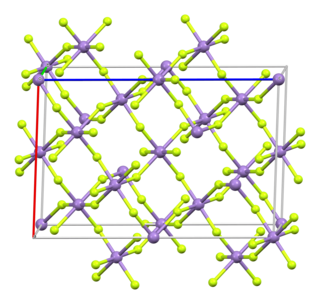
Manganese(III) fluoride (also known as Manganese trifluoride) is the inorganic compound with the formula MnF3. This red/purplish solid is useful for converting hydrocarbons into fluorocarbons, i.e., it is a fluorination agent. It forms a hydrate and many derivatives.

Oxygen fluorides are compounds of elements oxygen and fluorine with the general formula OnF2, where n = 1 to 6. Many different oxygen fluorides are known:
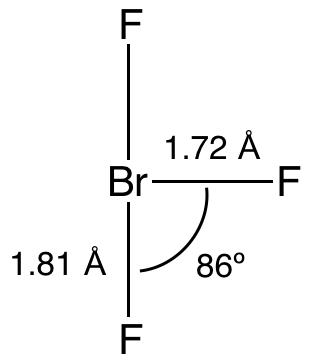
Bromine trifluoride is an interhalogen compound with the formula BrF3. At room temperature, it is a straw-coloured liquid with a pungent odor which decomposes violently on contact with water and organic compounds. It is a powerful fluorinating agent and an ionizing inorganic solvent. It is used to produce uranium hexafluoride (UF6) in the processing and reprocessing of nuclear fuel.

Nitrogen trifluoride is an inorganic, colorless, non-flammable, toxic gas with a slightly musty odor. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics. Nitrogen trifluoride is also an extremely strong and long-lived greenhouse gas. Its atmospheric burden exceeded 2 parts per trillion during 2019 and has doubled every five years since the late 20th century.

Silver(II) fluoride is a chemical compound with the formula AgF2. It is a rare example of a silver(II) compound - silver usually exists in its +1 oxidation state. It is used as a fluorinating agent.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.

Selenium compounds are compounds containing the element selenium (Se). Among these compounds, selenium has various oxidation states, the most common ones being −2, +4, and +6. Selenium compounds exist in nature in the form of various minerals, such as clausthalite, guanajuatite, tiemannite, crookesite etc., and can also coexist with sulfide minerals such as pyrite and chalcopyrite. For many mammals, selenium compounds are essential. For example, selenomethionine and selenocysteine are selenium-containing amino acids present in the human body. Selenomethionine participates in the synthesis of selenoproteins. The reduction potential and pKa (5.47) of selenocysteine are lower than those of cysteine, making some proteins have antioxidant activity. Selenium compounds have important applications in semiconductors, glass and ceramic industries, medicine, metallurgy and other fields.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.

Thiazyl fluoride, NSF, is a colourless, pungent gas at room temperature and condenses to a pale yellow liquid at 0.4 °C. Along with thiazyl trifluoride, NSF3, it is an important precursor to sulfur-nitrogen-fluorine compounds. It is notable for its extreme hygroscopicity.
A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In the nuclear industry, uranium hexafluoride (UF6) is an important intermediate in the purification of this element.

Thionyl tetrafluoride, also known as sulfur tetrafluoride oxide, is an inorganic compound with the formula SOF4. It is a colorless gas.
Iodine monofluoride is an interhalogen compound of iodine and fluorine with formula IF. It is a chocolate-brown solid that decomposes at 0 °C, disproportionating to elemental iodine and iodine pentafluoride:
Trifluorides are compounds in which one atom or ion has three fluorine atoms or ions associated. Many metals form trifluorides, such as iron, the rare-earth elements, and the metals in the groups 3, 13 and 15 of the periodic table. Most metal trifluorides are poorly soluble in water except ferric fluoride and indium(III) fluoride, but several are soluble in other solvents.

Thiophosphoryl fluoride is an inorganic molecular gas with formula PSF3 containing phosphorus, sulfur and fluorine. It spontaneously ignites in air and burns with a cool flame. The discoverers were able to have flames around their hands without discomfort, and called it "probably one of the coldest flames known". The gas was discovered in 1888.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
Thullium(III) fluoride is an inorganic compound with the chemical formula TmF3.

The hexafluoroarsenate anion is a chemical species with formula AsF−6. Hexafluoroarsenate is relatively inert, being the conjugate base of the notional superacid hexafluoroarsenic acid.











