
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated." The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's Rule. Aromatic compounds have the following general properties:

A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles.

Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the structural nucleus of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants.
Azoles are a class of five-membered heterocyclic compounds containing a nitrogen atom and at least one other non-carbon atom as part of the ring. Their names originate from the Hantzsch–Widman nomenclature. The parent compounds are aromatic and have two double bonds; there are successively reduced analogs with fewer. One, and only one, lone pair of electrons from each heteroatom in the ring is part of the aromatic bonding in an azole. Names of azoles maintain the prefix upon reduction. The numbering of ring atoms in azoles starts with the heteroatom that is not part of a double bond, and then proceeds towards the other heteroatom.
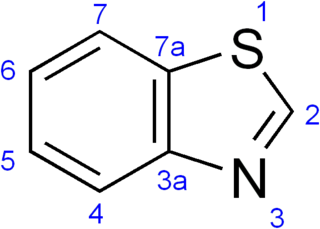
Benzothiazole, or more specifically 1,3-benzothiazole, is an aromatic heterocyclic compound with the chemical formula C
7H
5NS. It is colorless, slightly viscous liquid. Although the parent compound, benzothiazole is not widely used, many of its derivatives are found in commercial products or in nature. Firefly luciferin can be considered a derivative of benzothiazole.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.

Thioxanthene is a chemical compound in which the oxygen atom in xanthene is replaced with a sulfur atom. It is also related to phenothiazine. Several of its derivatives are used as typical antipsychotics in the treatment of schizophrenia and other psychoses.

Sulfur oxoacids are chemical compounds that contain sulfur, oxygen, and hydrogen. The best known and most important industrially used is sulfuric acid. Sulfur has several oxoacids; however, some of these are known only from their salts. The acids that have been characterised contain a variety of structural features, for example:

Oxazines are heterocyclic organic compounds containing one oxygen and one nitrogen atom in a cyclohexa-1,4-diene ring. Isomers exist depending on the relative position of the heteroatoms and relative position of the double bonds.

The benzhydryl compounds are a group of organic compounds whose parent structures include diphenylmethane, with any number of attached substituents, including bridges. This group typically excludes compounds in which either benzene is fused to another ring or includes a heteroatom, or where the methane connects to three or four benzenes.
Norsteroids are a structural class of steroids that have had an atom or atoms removed, biosynthetically or synthetically, from positions of branching off of rings or side chains, or from within rings of the steroid ring system. For instance, 19-norsteroids constitute an important class of natural and synthetic steroids derived by removal of the methyl group of the natural product progesterone; the equivalent change between testosterone and 19-nortestosterone (nandrolone) is illustrated below.

Dibenzazepine (iminostilbene) is a chemical compound with two benzene rings fused to an azepine ring. Many pharmaceuticals, such as carbamazepine, oxcarbazepine, and depramine, are based on a dibenzazepine structure.
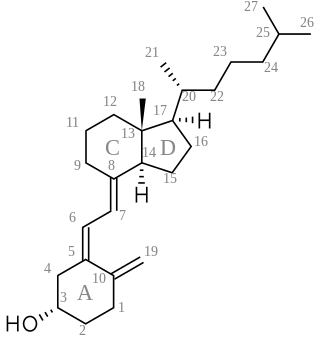
A secosteroid is a type of steroid with a "broken" ring. The word secosteroid derives from the Latin verb secare meaning "to cut", and 'steroid'. Secosteroids are described as a subclass of steroids under the IUPAC nomenclature. Some sources instead describe them as compounds derived from steroids.
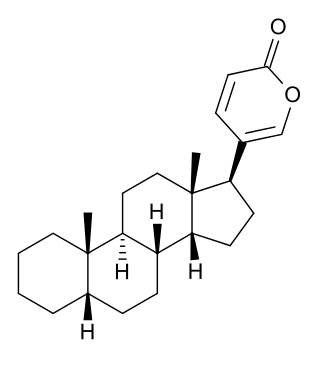
Bufadienolide is a chemical compound with steroid structure. Its derivatives are collectively known as bufadienolides, including many in the form of bufadienolide glycosides. These are a type of cardiac glycoside, the other being the cardenolide glycosides. Both bufadienolides and their glycosides are toxic; specifically, they can cause an atrioventricular block, bradycardia, ventricular tachycardia, and possibly lethal cardiac arrest.

Thiazepines are substituted thiepins, with a nitrogen replacing a carbon in the seven-membered heterocyclic compound. Depending on the location of the nitrogen, one distinguishes 1,3-thiazepine and 1,4-thiazepine.

Dibenzothiepins are chemical compounds which are derivatives of thiepin with two benzene rings.

Dibenzothiazepines are chemical compounds which are derivatives of thiazepine with two benzene rings.

Azocine is a heterocyclic organic compound with the molecular formula C7H7N. It consists of an unsaturated eight-membered ring having seven carbon atoms, one nitrogen atom and four double bonds.
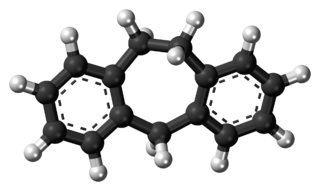
Dibenzocycloheptene is a tricyclic chemical compound featuring two benzene rings bound to a cycloheptene group. It is an occasional motif in synthetic organic chemistry. Various tricyclic antidepressants (TCAs) contain the dibenzocycloheptene moiety in their chemical structures, including amineptine, amitriptyline, amitriptylinoxide, butriptyline, demexiptiline, nortriptyline, noxiptiline, and protriptyline. Cyclobenzaprine, a skeletal muscle relaxant, also contains this functional group.
5-methyltetrahydrofolate:corrinoid/iron-sulfur protein Co-methyltransferase is an enzyme with systematic name 5-methyltetrahydrofolate:corrinoid/iron-sulfur protein methyltransferase. This enzyme catalyses the following chemical reaction



















