
Desalination is a process that takes away mineral components from saline water. More generally, desalination refers to the removal of salts and minerals from a target substance, as in soil desalination, which is an issue for agriculture. Saltwater is desalinated to produce water suitable for human consumption or irrigation. The by-product of the desalination process is brine. Desalination is used on many seagoing ships and submarines. Most of the modern interest in desalination is focused on cost-effective provision of fresh water for human use. Along with recycled wastewater, it is one of the few rainfall-independent water resources.

Semipermeable membrane is a type of biological or synthetic, polymeric membrane that will allow certain molecules or ions to pass through it by osmosis. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials which are rather thick are also semipermeable. One example of this is the thin film on the inside of the egg.
Ultrafiltration (UF) is a variety of membrane filtration in which forces such as pressure or concentration gradients lead to a separation through a semipermeable membrane. Suspended solids and solutes of high molecular weight are retained in the so-called retentate, while water and low molecular weight solutes pass through the membrane in the permeate (filtrate). This separation process is used in industry and research for purifying and concentrating macromolecular (103–106 Da) solutions, especially protein solutions.

Forward osmosis (FO) is an osmotic process that, like reverse osmosis (RO), uses a semi-permeable membrane to effect separation of water from dissolved solutes. The driving force for this separation is an osmotic pressure gradient, such that a "draw" solution of high concentration, is used to induce a net flow of water through the membrane into the draw solution, thus effectively separating the feed water from its solutes. In contrast, the reverse osmosis process uses hydraulic pressure as the driving force for separation, which serves to counteract the osmotic pressure gradient that would otherwise favor water flux from the permeate to the feed. Hence significantly more energy is required for reverse osmosis compared to forward osmosis.

Gas mixtures can be effectively separated by synthetic membranes made from polymers such as polyamide or cellulose acetate, or from ceramic materials.
Solar desalination is a desalination technique powered by solar energy. The two common methods are direct (thermal) and indirect (photovoltaic).
Barrer is a non-SI unit of gas permeability used in the membrane technology and contact lens industry. It is named after Richard Barrer.

Menachem Elimelech is the Sterling Professor of Chemical and Environmental Engineering at Yale University. Elimelech is the only professor from an engineering department at Yale to be awarded the Sterling professorship since its establishment in 1920. Elimelech moved from the University of California, Los Angeles (UCLA) to Yale University in 1998 and founded Yale's Environmental Engineering program.
Nanofiltration is a membrane filtration process used most often to soften and disinfect water.

Membrane fouling is a process whereby a solution or a particle is deposited on a membrane surface or in membrane pores in a processes such as in a membrane bioreactor, reverse osmosis, forward osmosis, membrane distillation, ultrafiltration, microfiltration, or nanofiltration so that the membrane's performance is degraded. It is a major obstacle to the widespread use of this technology. Membrane fouling can cause severe flux decline and affect the quality of the water produced. Severe fouling may require intense chemical cleaning or membrane replacement. This increases the operating costs of a treatment plant. There are various types of foulants: colloidal, biological, organic and scaling.
Reverse osmosis (RO) is a water purification process that uses a semi-permeable membrane to separate water molecules from other substances. RO applies pressure to overcome osmotic pressure that favors even distributions. RO can remove dissolved or suspended chemical species as well as biological substances, and is used in industrial processes and the production of potable water. RO retains the solute on the pressurized side of the membrane and the purified solvent passes to the other side. It relies on the relative sizes of the various molecules to decide what passes through. "Selective" membranes reject large molecules, while accepting smaller molecules.

Pressure retarded osmosis (PRO) is a technique to separate a solvent from a solution that is more concentrated and also pressurized. A semipermeable membrane allows the solvent to pass to the concentrated solution side by osmosis. The technique can be used to generate power from the salinity gradient energy resulting from the difference in the salt concentration between sea and river water. In PRO, the water potential between fresh water and sea water corresponds to a pressure of 26 bars. This pressure is equivalent to a column of water 270 meters high. However, the optimal working pressure is only half of this, 11 to 15 bar.

A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. Biological membranes include cell membranes ; nuclear membranes, which cover a cell nucleus; and tissue membranes, such as mucosae and serosae. Synthetic membranes are made by humans for use in laboratories and industry.
Membrane distillation (MD) is a thermally driven separation process in which separation is driven by phase change. A hydrophobic membrane presents a barrier for the liquid phase, allowing the vapour phase to pass through the membrane's pores. The driving force of the process is a partial vapour pressure difference commonly triggered by a temperature difference.

Interfacial polymerization is a type of step-growth polymerization in which polymerization occurs at the interface between two immiscible phases, resulting in a polymer that is constrained to the interface. There are several variations of interfacial polymerization, which result in several types of polymer topologies, such as ultra-thin films, nanocapsules, and nanofibers, to name just a few.
Water shortages have become an increasingly pressing concern recently and with recent predictions of a high probability of the current drought turning into a megadrought occurring in the western United States, technologies involving water treatment and processing need to improve. Carbon nanotubes (CNT) have been the subject of extensive studies because they demonstrate a range of unique properties that existing technologies lack. For example, carbon nanotube membranes can demonstrate higher water flux with lower energy than current membranes. These membranes can also filter out particles that are too small for conventional systems which can lead to better water purification techniques and less waste. The largest obstacle facing CNT is processing as it is difficult to produce them in the large quantities that most of these technologies will require.
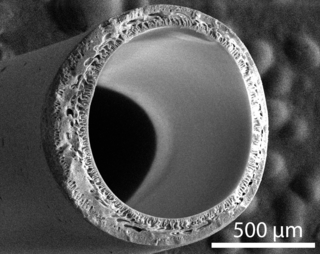
Hollow fiber membranes (HFMs) are a class of artificial membranes containing a semi-permeable barrier in the form of a hollow fiber. Originally developed in the 1960s for reverse osmosis applications, hollow fiber membranes have since become prevalent in water treatment, desalination, cell culture, medicine, and tissue engineering. Most commercial hollow fiber membranes are packed into cartridges which can be used for a variety of liquid and gaseous separations.
Phase inversion or phase separation is a chemical phenomenon exploited in the fabrication of artificial membranes. It is performed by removing the solvent from a liquid-polymer solution, leaving a porous, solid membrane.

Cardo polymers are a sub group of polymers where ring structures are pendent to the polymer backbone. The backbone carbons bonded to the pendent ring structures are quaternary centers. As such, the cyclic side group lies perpendicular to the plane of the extended polymer chain. These side rings are bulky structures which sterically hinder rotation of the backbone bonds; they also disrupt chain packing and thus create greater free volume than found in conventional polymer structures. The rotational restrictions placed on the polymer backbone increase the value of the characteristic ratio, leading to what is referred to as a "rigid" or "stiff" backbone. The sterically driven large rotational potential produces a high glass transition temperature and the disrupted packing yields comparatively high values for gas and other small molecule solubilities in these polymers. Because of these physical effects, recent advances in membranes used for gas separation have used cardo polymers.
A zeolite membrane is a synthetic membrane made of crystalline aluminosilicate materials, typically aluminum, silicon, and oxygen with positive counterions such as Na+ and Ca2+ within the structure. Zeolite membranes serve as a low energy separation method. They have recently drawn interest due to their high chemical and thermal stability, and their high selectivity. Currently zeolites have seen applications in gas separation, membrane reactors, water desalination, and solid state batteries. Currently zeolite membranes have yet to be widely implemented commercially due to key issues including low flux, high cost of production, and defects in the crystal structure.










