
The Actinomycetota are a phylum of mostly Gram-positive bacteria. They can be terrestrial or aquatic. They are of great economic importance to humans because agriculture and forests depend on their contributions to soil systems. In soil they help to decompose the organic matter of dead organisms so the molecules can be taken up anew by plants. While this role is also played by fungi, Actinomycetota are much smaller and likely do not occupy the same ecological niche. In this role the colonies often grow extensive mycelia, like a fungus would, and the name of an important order of the phylum, Actinomycetales, reflects that they were long believed to be fungi. Some soil actinomycetota live symbiotically with the plants whose roots pervade the soil, fixing nitrogen for the plants in exchange for access to some of the plant's saccharides. Other species, such as many members of the genus Mycobacterium, are important pathogens.
Polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone (or reduced forms of a ketone) and methylene groups: (-CO-CH2-). First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or better bioactivity.
Topoisomerase inhibitors are chemical compounds that block the action of topoisomerases, which are broken into two broad subtypes: type I topoisomerases (TopI) and type II topoisomerases (TopII). Topoisomerase plays important roles in cellular reproduction and DNA organization, as they mediate the cleavage of single and double stranded DNA to relax supercoils, untangle catenanes, and condense chromosomes in eukaryotic cells. Topoisomerase inhibitors influence these essential cellular processes. Some topoisomerase inhibitors prevent topoisomerases from performing DNA strand breaks while others, deemed topoisomerase poisons, associate with topoisomerase-DNA complexes and prevent the re-ligation step of the topoisomerase mechanism. These topoisomerase-DNA-inhibitor complexes are cytotoxic agents, as the un-repaired single- and double stranded DNA breaks they cause can lead to apoptosis and cell death. Because of this ability to induce apoptosis, topoisomerase inhibitors have gained interest as therapeutics against infectious and cancerous cells.
A depsipeptide is a peptide in which one or more of its amide, -C(O)NHR-, groups are replaced by the corresponding ester, -C(O)OR, Many depsipeptides have both peptide and ester linkages. They are mainly found in marine and microbial natural products.

Cryptophycins are a family of macrolide molecules that are potent cytotoxins and have been studied for potential antiproliferative properties useful in developing chemotherapy. They are members of the depsipeptide family.
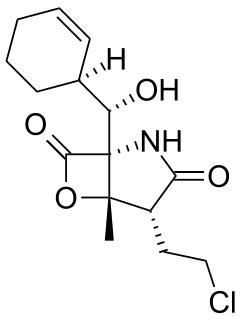
Salinosporamide A (Marizomib) is a potent proteasome inhibitor being studied as a potential anticancer agent. It entered phase I human clinical trials for the treatment of multiple myeloma, only three years after its discovery in 2003. This marine natural product is produced by the obligate marine bacteria Salinispora tropica and Salinispora arenicola, which are found in ocean sediment. Salinosporamide A belongs to a family of compounds, known collectively as salinosporamides, which possess a densely functionalized γ-lactam-β-lactone bicyclic core.

Indolocarbazoles (ICZs) are a class of compounds that are under current study due to their potential as anti-cancer drugs and the prospective number of derivatives and uses found from the basic backbone alone. First isolated in 1977, a wide range of structures and derivatives have been found or developed throughout the world. Due to the extensive number of structures available, this review will focus on the more important groups here while covering their occurrence, biological activity, biosynthesis, and laboratory synthesis.
Streptogramin A is a group of antibiotics within the larger family of antibiotics known as streptogramins. They are synthesized by the bacteria Streptomyces virginiae. The streptogramin family of antibiotics consists of two distinct groups: group A antibiotics contain a 23-membered unsaturated ring with lactone and peptide bonds while group B antibiotics are depsipeptides. While structurally different, these two groups of antibiotics act synergistically, providing greater antibiotic activity than the combined activity of the separate components. These antibiotics have until recently been commercially manufactured as feed additives in agriculture, although today there is increased interest in their ability to combat antibiotic-resistant bacteria, particularly vancomycin-resistant bacteria.

Romidepsin, also known as Istodax, is an anticancer agent used in cutaneous T-cell lymphoma (CTCL) and other peripheral T-cell lymphomas (PTCLs). Romidepsin is a natural product obtained from the bacterium Chromobacterium violaceum, and works by blocking enzymes known as histone deacetylases, thus inducing apoptosis. It is sometimes referred to as depsipeptide, after the class of molecules to which it belongs. Romidepsin is branded and owned by Gloucester Pharmaceuticals, now a part of Celgene.
Bohemic acid is a mixture of chemical compounds which is obtained through fermentation by actinobacteria species in the genus Actinosporangium (Actinoplanaceae). The name honors the Puccini opera La Bohème and many individual components of the acid carry the names of characters from La Bohème. Most of those components are antitumor agents and anthracycline antibiotics active against Gram-positive bacteria.
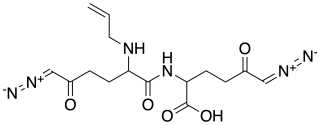
Alazopeptin is an antibiotic, with moderate anti-trypanosomal and antitumor activity. It was originally isolated from Streptacidiphilus griseoplanus, sourced from soil near Williamsburg, Iowa. It is also isolated from Kitasatospora azatica. It is still largely produced via fermentation broths of that organism. Structurally, alazopeptin is a tripeptide and contains 2 molecules of 6-diazo-5-oxo-L-norleucine and one molecule of L-alanine. In 2021 the biosynthetic pathway of alazopeptin was elucidated.

Callystatin A is a polyketide natural product from the leptomycin family of secondary metabolites. It was first isolated in 1997 from the marine sponge Callyspongia truncata which was collected from the Goto Islands in the Nagasaki Prefecture of Japan by the Kobayashi group. Since then its absolute configuration has been elucidated and callystatin A was discovered to have anti-fungal and anti-tumor activities with extreme potency against the human epidermoid carcinoma KB cells (IG50 = 10 pg/ml) and the mouse lymphocytic leukemia Ll210 cells (IG50 = 20 pg/ml).
Altemicidin is monoterpene alkaloid first identified in isolates from marine actinomycetes in 1989. It may also be produced synthetically. Altemicidin displays both acaricidal and antitumor activity.
Marinone is an antibiotic made by marine actinomycetes.

Salinispora is a genus of obligately aerobic, gram-positive, non-acid-fast bacteria belonging to the family of Micromonosporaceae. They are heterotrophic, non-motile, and obligately grow under high osmotic/ionic-strength conditions. They are the first identified genus of gram-positive bacteria which has a high osmotic/ionic-strength requirement for survival. They are widely abundant in tropical marine sediments and were first identified in 2002. This genus of bacteria has potential biotechnological significance due to their production of novel secondary metabolites which can be used pharmaceutically.
Penicillium herquei is an anamorph, filamentous species of the genus of Penicillium which produces citreorosein, emodin, hualyzin, herquline B, janthinone, citrinin and duclauxin,.
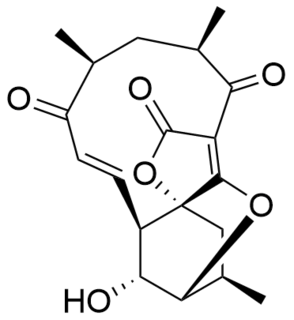
Atrop-abyssomicin C is a polycyclic polyketide-type natural product that is the atropisomer of abyssomicin C. It is a spirotetronate that belongs to the class of tetronate antibiotics, which includes compounds such as tetronomycin, agglomerin, and chlorothricin. In 2006, the Nicolaou group discovered atrop-abyssomicin C while working on the total synthesis of abyssomicin C. Then in 2007, Süssmuth and co-workers isolated atrop-abyssomicin C from Verrucosispora maris AB-18-032, a marine actinomycete found in sediment of the Japanese sea. They found that atrop-abyssomicin C was the major metabolite produced by this strain, while abyssomicin C was a minor product. The molecule displays antibacterial activity by inhibiting the enzyme PabB, thereby depleting the biosynthesis of p-aminobenzoate.

Pentabromopseudilin, the first reported marine microbial antibiotic, is a bioactive natural product that contains a highly halogenated 2-arrylpyrrole moiety. Pentabromopseudilin (PBP) is a unique hybrid bromophenol-bromopyrrole compound that is made up of over 70% bromine atoms, contributing to its potent bioactivity. PBP was first isolated from Pseudomonas bromoutilis, and has since been found to be produced by other marine microbes, including Alteromonas luteoviolaceus, Chromobacteria, and Pseudoalteromonas spp.

C-1027 or Lidamycin is an antitumor antibiotic consisting of a complex of an enediyne chromophore and an apoprotein. It shows antibiotic activity against most Gram-positive bacteria. It is one of the most potent cytotoxic molecules known, due to its induction of a higher ratio of DNA double-strand breaks than single-strand breaks.
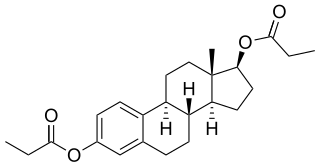
Estradiol dipropionate/hydroxyprogesterone caproate (EDP/OHPC), sold under the brand name EP Hormone Depot, is a combined estrogen–progestogen medication which is used in Japan. It is manufactured by Teikoku Zoki Pharmaceutical Co., Tokyo and contains 1 mg/mL estradiol dipropionate and 50 mg/mL hydroxyprogesterone caproate.














