
Thiolactones are a class of heterocyclic compounds in organic chemistry. They are analogs of the more common lactones in which an oxygen atom is replaced with a sulfur atom. The sulfur atom is within the ring system and adjacent to a carbonyl group.

Thiolactones are a class of heterocyclic compounds in organic chemistry. They are analogs of the more common lactones in which an oxygen atom is replaced with a sulfur atom. The sulfur atom is within the ring system and adjacent to a carbonyl group.
Thiolactones can be prepared by dehydration of thiol-containing carboxylic acids. Thiolactones can be hydrolyzed back to the thiol acids under basic conditions. [1] β-Thiolactones can be opened by reaction at the 4-position via SN2 nucleophilic reactions. [2]
The most common thiolactone, homocysteine thiolactone is produced biochemically from homocysteine and it may play a role in protein damage. [3] The thiolactone functional group is also present in some pharmaceutical drugs such as citiolone and erdosteine. Thiolactone rings can also be found in peptides synthesized by bacteria such as Staphylococcus aureus in order to regulate their quorum-sensing system. [4]

In chemistry, an ester is a chemical compound derived from an acid in which at least one –OH (hydroxyl) group is replaced by an –O–alkyl (alkoxy) group. Usually, esters are derived from substitution reaction of a carboxylic acid and an alcohol. Glycerides, which are fatty acid esters of glycerol, are important esters in biology, being one of the main classes of lipids, and making up the bulk of animal fats and vegetable oils. Esters with low molecular weight are commonly used as fragrances and found in essential oils and pheromones. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties, while polyesters are important plastics, with monomers linked by ester moieties. Esters usually have a sweet smell and are considered high-quality solvents for a broad array of plastics, plasticizers, resins, and lacquers. They are also one of the largest classes of synthetic lubricants on the commercial market.

Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula HOOC-CH-(NH2)-CH2-SH. The thiol side chain in cysteine often participates in enzymatic reactions, as a nucleophile. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. When used as a food additive, it has the E number E920. It is encoded by the codons UGU and UGC.

Methionine is an essential amino acid in humans. As the substrate for other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical role in the metabolism and health of many species, including humans. It is encoded by the codon AUG.
In chemistry, a disulfide refers to a functional group with the structure R−S−S−R′. The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. The connection is a persulfide, in analogy to its congener, peroxide (R−O−O−R′), but this terminology is rarely used, except in reference to hydrodisulfides.
The following outline is provided as an overview of and topical guide to organic chemistry:
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure, or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring.
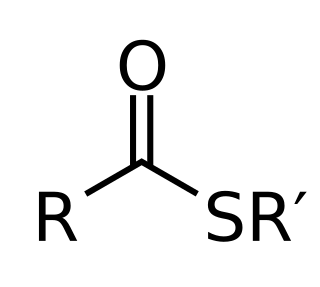
In chemistry thioesters are compounds with the functional group R–S–CO–R'. They are analogous to carboxylate esters with the sulfur in the thioester playing the role of the linking oxygen in the carboxylate ester. They are the product of esterification between a carboxylic acid and a thiol. In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group -COCl. Their formula is usually written RCOCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.
Native chemical ligation or NCL is an important extension of the chemical ligation field, a concept for constructing a large polypeptide formed by the assembling of two or more unprotected peptides segments. Especially, NCL is the most powerful ligation method for synthesizing native backbone proteins or modified proteins of moderate size.
A lactam is a cyclic amide. The term is a portmanteau of the words lactone + amide.
The Ugi reaction is a multi-component reaction in organic chemistry involving a ketone or aldehyde, an amine, an isocyanide and a carboxylic acid to form a bis-amide. The reaction is named after Ivar Karl Ugi, who first reported this reaction in 1959.
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone. It is named after Rainer Ludwig Claisen, who first published his work on the reaction in 1887.

Dithiothreitol (DTT) is the common name for a small-molecule redox reagent also known as Cleland's reagent. DTT's formula is C4H10O2S2 and the chemical structure of one of its enantiomers in its reduced form is shown on the right; its oxidized form is a disulfide bonded 6-membered ring (shown below). The reagent is commonly used in its racemic form, as both enantiomers are reactive. Its name derives from the four-carbon sugar, threose. DTT has an epimeric ('sister') compound, dithioerythritol (DTE).

The Robinson–Gabriel synthesis is an organic reaction in which a 2-acylamino-ketone reacts intramolecularly followed by a dehydration to give an oxazole. A cyclodehydrating agent is needed to catalyze the reaction It is named after Sir Robert Robinson and Siegmund Gabriel who described the reaction in 1909 and 1910, respectively.
Organoselenium compounds are chemical compounds containing carbon-to-selenium chemical bonds. Organoselenium chemistry is the corresponding science exploring their properties and reactivity. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected.

Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenols except the oxygen atom in the hydroxyl group (-OH) bonded to the aromatic ring is replaced by a sulfur atom. The prefix thio- implies a sulfur-containing compound and when used before a root word name for a compound which would normally contain an oxygen atom, in the case of 'thiol' that the alcohol oxygen atom is replaced by a sulfur atom.
Cyanogen bromide is the inorganic compound with the formula (CN)Br or BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides, and synthesize other compounds. The compound is classified as a pseudohalogen.
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen end to an organosilicon group. They are important intermediates in organic synthesis.
Shiina macrolactonization is an organic chemical reaction that synthesizes cyclic compounds by using aromatic carboxylic acid anhydrides as dehydration condensation agents. In 1994, Prof. Isamu Shiina reported an acidic cyclization method using Lewis acid catalyst, and, in 2002, a basic cyclization using nucleophilic catalyst.
Shiina esterification is an organic chemical reaction that synthesizes carboxylic esters from nearly equal amounts of carboxylic acids and alcohols by using aromatic carboxylic acid anhydrides as dehydration condensation agents. In 1994, Prof. Isamu Shiina reported an acidic coupling method using Lewis acid, and, in 2002, a basic esterification using nucleophilic catalyst.