Autocrine signaling is a form of cell signaling in which a cell secretes a hormone or chemical messenger that binds to autocrine receptors on that same cell, leading to changes in the cell. This can be contrasted with paracrine signaling, intracrine signaling, or classical endocrine signaling.

Catenins are a family of proteins found in complexes with cadherin cell adhesion molecules of animal cells. The first two catenins that were identified became known as α-catenin and β-catenin. α-Catenin can bind to β-catenin and can also bind filamentous actin (F-actin). β-Catenin binds directly to the cytoplasmic tail of classical cadherins. Additional catenins such as γ-catenin and δ-catenin have been identified. The name "catenin" was originally selected because it was suspected that catenins might link cadherins to the cytoskeleton.
The epithelial–mesenchymal transition (EMT) is a process by which epithelial cells lose their cell polarity and cell–cell adhesion, and gain migratory and invasive properties to become mesenchymal stem cells; these are multipotent stromal cells that can differentiate into a variety of cell types. EMT is essential for numerous developmental processes including mesoderm formation and neural tube formation. EMT has also been shown to occur in wound healing, in organ fibrosis and in the initiation of metastasis in cancer progression.

Cardiotoxin III is a sixty amino-acid polypeptide toxin from the Taiwan cobra Naja atra. CTX III is highly basic and hydrophobic protein. It is an example of a group of snake cardio/cytotoxins, which are made up of shorter snake venom three-finger toxins. Over 50 different cytotoxin polypeptides have been isolated and sequenced from venom samples. The difference in the CTX functionality may be due to the relatively small difference in the polypeptide's structure, allowing different CTXs to induce lysis in different cell types. The CTX III molecule contains multiple binding sites and is cytolytic for myocardial cells and human leukemic T cells.

Sorafenib, sold under the brand name Nexavar, is a kinase inhibitor drug approved for the treatment of primary kidney cancer, advanced primary liver cancer, FLT3-ITD positive AML and radioactive iodine resistant advanced thyroid carcinoma.
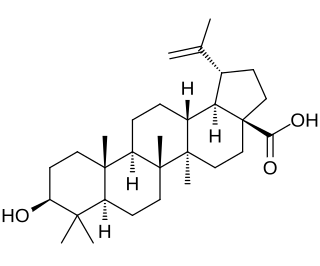
Betulinic acid is a naturally occurring pentacyclic triterpenoid which has antiretroviral, antimalarial, and anti-inflammatory properties, as well as a more recently discovered potential as an anticancer agent, by inhibition of topoisomerase. It is found in the bark of several species of plants, principally the white birch from which it gets its name, but also the ber tree, selfheal, the tropical carnivorous plants Triphyophyllum peltatum and Ancistrocladus heyneanus, Diospyros leucomelas, a member of the persimmon family, Tetracera boiviniana, the jambul, flowering quince, rosemary, and Pulsatilla chinensis.

FAS-associated death domain protein, also called MORT1, is encoded by the FADD gene on the 11q13.3 region of chromosome 11 in humans.

Tropolone is an organic compound with the chemical formula C7H5(OH)O. It is a pale yellow solid that is soluble in organic solvents. The compound has been of interest to research chemists because of its unusual electronic structure and its role as a ligand precursor. Although not usually prepared from tropone, it can be viewed as its derivative with a hydroxyl group in the 2-position.

Peptidylprolyl isomerase A (PPIA), also known as cyclophilin A (CypA) or rotamase A is an enzyme that in humans is encoded by the PPIA gene on chromosome 7. As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the cis-trans isomerization of proline imidic peptide bonds, which allows it to regulate many biological processes, including intracellular signaling, transcription, inflammation, and apoptosis. Due to its various functions, PPIA has been implicated in a broad range of inflammatory diseases, including atherosclerosis and arthritis, and viral infections.
The estrogen receptor test (ERT) is a laboratory test to determine whether cancer cells have estrogen receptors. This information can guide treatment of the cancer.

Retrotransposon-derived protein PEG10 is a protein that in humans is encoded by the PEG10 gene.

SET and MYND (myeloid-Nervy-DEAF-1) domain-containing protein 3 is a protein that in humans is encoded by the SMYD3 gene.

Arenobufagin is a cardiotoxic bufanolide steroid secreted by the Argentine toad Bufo arenarum. It has effects similar to digitalis, blocking the Na+/K+ pump in heart tissue.

Activin and inhibin are two closely related protein complexes that have almost directly opposite biological effects. Identified in 1986, activin enhances FSH biosynthesis and secretion, and participates in the regulation of the menstrual cycle. Many other functions have been found to be exerted by activin, including roles in cell proliferation, differentiation, apoptosis, metabolism, homeostasis, immune response, wound repair, and endocrine function. Conversely, inhibin downregulates FSH synthesis and inhibits FSH secretion. The existence of inhibin was hypothesized as early as 1916; however, it was not demonstrated to exist until Neena Schwartz and Cornelia Channing's work in the mid-1970s, after which both proteins were molecularly characterized ten years later.

Thujaplicin is any of three isomeric tropolone-related natural products that have been isolated from the softwoods of the trees of Cupressaceae family. These compounds are known for their antibacterial, antifungal, and antioxidant properties. They were the first natural tropolones to be made synthetically.

Hinokitiol (β-thujaplicin) is a natural monoterpenoid found in the wood of trees in the family Cupressaceae. It is a tropolone derivative and one of the thujaplicins. Hinokitiol is used in oral and skin care products, and is a food additive used in Japan.

In molecular biology mir-22 microRNA is a short RNA molecule. MicroRNAs are an abundant class of molecules, approximately 22 nucleotides in length, which can post-transcriptionally regulate gene expression by binding to the 3' UTR of mRNAs expressed in a cell.
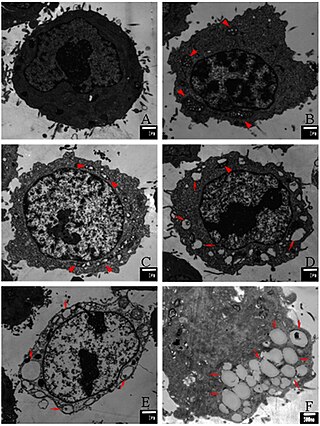
Paraptosis is a type of programmed cell death, morphologically distinct from apoptosis and necrosis. The defining features of paraptosis are cytoplasmic vacuolation, independent of caspase activation and inhibition, and lack of apoptotic morphology. Paraptosis lacks several of the hallmark characteristics of apoptosis, such as membrane blebbing, chromatin condensation, and nuclear fragmentation. Like apoptosis and other types of programmed cell death, the cell is involved in causing its own death, and gene expression is required. This is in contrast to necrosis, which is non-programmed cell death that results from injury to the cell.

Histidine triad nucleotide binding protein 2 (HINT2) is a mitochondrial protein that in humans is encoded by the HINT2 gene on chromosome 9. This protein is an AMP-lysine hydrolase and phosphoamidase and may contribute to tumor suppression.

















