
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 °C (237 °F), and boils to a violet gas at 184 °C (363 °F). The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek Ιώδης 'violet-coloured'.

Silver iodide is an inorganic compound with the formula AgI. The compound is a bright yellow solid, but samples almost always contain impurities of metallic silver that give a gray coloration. The silver contamination arises because AgI is highly photosensitive. This property is exploited in silver-based photography. Silver iodide is also used as an antiseptic and in cloud seeding.
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability.

Lead(II) iodide is a chemical compound with the formula PbI
2. At room temperature, it is a bright yellow odorless crystalline solid, that becomes orange and red when heated. It was formerly called plumbous iodide.

Potassium iodide is a chemical compound, medication, and dietary supplement. It is a medication used for treating hyperthyroidism, in radiation emergencies, and for protecting the thyroid gland when certain types of radiopharmaceuticals are used. In the third world it is also used for treating skin sporotrichosis and phycomycosis. It is a supplement used by people with low dietary intake of iodine. It is administered orally.

Phosphorus triiodide (PI3) is an inorganic compound with the formula PI3. A red solid, it is a common misconception that PI3 is too unstable to be stored; it is, in fact, commercially available. It is widely used in organic chemistry for converting alcohols to alkyl iodides. It is also a powerful reducing agent. Note that phosphorus also forms a lower iodide, P2I4, but the existence of PI5 is doubtful at room temperature.

Hydrogen iodide is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under standard conditions, whereas the other is an aqueous solution of the gas. They are interconvertible. HI is used in organic and inorganic synthesis as one of the primary sources of iodine and as a reducing agent.

Caesium iodide or cesium iodide is the ionic compound of caesium and iodine. It is often used as the input phosphor of an X-ray image intensifier tube found in fluoroscopy equipment. Caesium iodide photocathodes are highly efficient at extreme ultraviolet wavelengths.

Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions (I−) in a crystal lattice. It is used mainly as a nutritional supplement and in organic chemistry. It is produced industrially as the salt formed when acidic iodides react with sodium hydroxide. It is a chaotropic salt.

Lithium iodide, or LiI, is a compound of lithium and iodine. When exposed to air, it becomes yellow in color, due to the oxidation of iodide to iodine. It crystallizes in the NaCl motif. It can participate in various hydrates.

Copper(I) iodide is the inorganic compound with the formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding.

Mercury(II) iodide is a chemical compound with the molecular formula HgI2. It is typically produced synthetically but can also be found in nature as the extremely rare mineral coccinite. Unlike the related mercury(II) chloride it is hardly soluble in water (<100 ppm).

Magnesium iodide is the name for the chemical compounds with the formulas MgI2 and its various hydrates MgI2(H2O)x. These salts are typical ionic halides, being highly soluble in water.
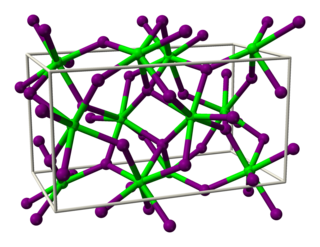
Strontium iodide (SrI2) is a salt of strontium and iodine. It is an ionic, water-soluble, and deliquescent compound that can be used in medicine as a substitute for potassium iodide . It is also used as a scintillation gamma radiation detector, typically doped with europium, due to its optical clarity, relatively high density, high effective atomic number (Z=48), and high scintillation light yield. In recent years, europium-doped strontium iodide (SrI2:Eu2+) has emerged as a promising scintillation material for gamma-ray spectroscopy with extremely high light yield and proportional response, exceeding that of the widely used high performance commercial scintillator LaBr3:Ce3+. Large diameter SrI2 crystals can be grown reliably using vertical Bridgman technique and are being commercialized by several companies.
Organoiodine compounds are organic compounds that contain one or more carbon–iodine bonds. They occur widely in organic chemistry, but are relatively rare in nature. The thyroxine hormones are organoiodine compounds that are required for health and the reason for government-mandated iodization of salt.

Manganese(II) iodide is the chemical compound composed of manganese and iodide with the formula MnI2. The anhydrous compound adopts the cadmium iodide crystal structure. The pink tetrahydrate is known. Unlike MnX2(H2O)4, which are cis for X = Cl, Br, the iodides are trans in MnI2(H2O)4.
Iron(II) iodide is an inorganic compound with the chemical formula FeI2. It is used as a catalyst in organic reactions.

Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound.

Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3.
















