Related Research Articles

An alloy is a mixture of chemical elements of which in most cases at least one is a metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Most alloys are metallic and show good electrical conductivity, ductility, opacity, and luster, and may have properties that differ from those of the pure elements such as increased strength or hardness. In some cases, an alloy may reduce the overall cost of the material while preserving important properties. In other cases, the mixture imparts synergistic properties such as corrosion resistance or mechanical strength.

A metal is a material that, when polished or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. These properties are all associated with having electrons available at the Fermi level, as against nonmetallic materials which do not. Metals are typically ductile and malleable.

Solder is a fusible metal alloy used to create a permanent bond between metal workpieces. Solder is melted in order to wet the parts of the joint, where it adheres to and connects the pieces after cooling. Metals or alloys suitable for use as solder should have a lower melting point than the pieces to be joined. The solder should also be resistant to oxidative and corrosive effects that would degrade the joint over time. Solder used in making electrical connections also needs to have favorable electrical characteristics.

Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in sea water, aqua regia, and chlorine.

Heat treating is a group of industrial, thermal and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are also used in the manufacture of many other materials, such as glass. Heat treatment involves the use of heating or chilling, normally to extreme temperatures, to achieve the desired result such as hardening or softening of a material. Heat treatment techniques include annealing, case hardening, precipitation strengthening, tempering, carburizing, normalizing and quenching. Although the term heat treatment applies only to processes where the heating and cooling are done for the specific purpose of altering properties intentionally, heating and cooling often occur incidentally during other manufacturing processes such as hot forming or welding.

Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states:

An intermetallic is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic elements. Intermetallics are generally hard and brittle, with good high-temperature mechanical properties. They can be classified as stoichiometric or nonstoichiometic intermetallic compounds.

Maraging steels are steels that are known for possessing superior strength and toughness without losing ductility. Aging refers to the extended heat-treatment process. These steels are a special class of very-low-carbon ultra-high-strength steels that derive their strength not from carbon, but from precipitation of intermetallic compounds. The principal alloying element is 15 to 25 wt% nickel. Secondary alloying elements, which include cobalt, molybdenum and titanium, are added to produce intermetallic precipitates. Original development was carried out on 20 and 25 wt% Ni steels to which small additions of aluminium, titanium, and niobium were made; a rise in the price of cobalt in the late 1970s led to the development of cobalt-free maraging steels.

Tempering is a process of heat treating, which is used to increase the toughness of iron-based alloys. Tempering is usually performed after hardening, to reduce some of the excess hardness, and is done by heating the metal to some temperature below the critical point for a certain period of time, then allowing it to cool in still air. The exact temperature determines the amount of hardness removed, and depends on both the specific composition of the alloy and on the desired properties in the finished product. For instance, very hard tools are often tempered at low temperatures, while springs are tempered at much higher temperatures.

Titanium nitride is an extremely hard ceramic material, often used as a physical vapor deposition (PVD) coating on titanium alloys, steel, carbide, and aluminium components to improve the substrate's surface properties.

Titanium alloys are alloys that contain a mixture of titanium and other chemical elements. Such alloys have very high tensile strength and toughness. They are light in weight, have extraordinary corrosion resistance and the ability to withstand extreme temperatures. However, the high cost of processing limits their use to military applications, aircraft, spacecraft, bicycles, medical devices, jewelry, highly stressed components such as connecting rods on expensive sports cars and some premium sports equipment and consumer electronics.
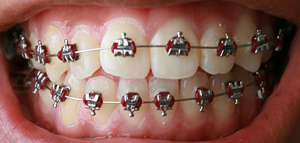
An archwire in orthodontics is a wire conforming to the alveolar or dental arch that can be used with dental braces as a source of force in correcting irregularities in the position of the teeth. An archwire can also be used to maintain existing dental positions; in this case it has a retentive purpose.
Titanium aluminide, commonly gamma titanium, is an intermetallic chemical compound. It is lightweight and resistant to oxidation and heat, but has low ductility. The density of γ-TiAl is about 4.0 g/cm3. It finds use in several applications including aircraft, jet engines, sporting equipment and automobiles. The development of TiAl based alloys began circa 1970. The alloys have been used in these applications only since about 2000.

Alloy steel is steel that is alloyed with a variety of elements in amounts between 1.0% and 50% by weight, typically to improve its mechanical properties.
In metallurgy, solid solution strengthening is a type of alloying that can be used to improve the strength of a pure metal. The technique works by adding atoms of one element to the crystalline lattice of another element, forming a solid solution. The local nonuniformity in the lattice due to the alloying element makes plastic deformation more difficult by impeding dislocation motion through stress fields. In contrast, alloying beyond the solubility limit can form a second phase, leading to strengthening via other mechanisms.
Nickel aluminide refers to either of two widely used intermetallic compounds, Ni3Al or NiAl, but the term is sometimes used to refer to any nickel–aluminium alloy. These alloys are widely used because of their high strength even at high temperature, low density, corrosion resistance, and ease of production. Ni3Al is of specific interest as a precipitate in nickel-based superalloys, where it is called the γ' (gamma prime) phase. It gives these alloys high strength and creep resistance up to 0.7–0.8 of its melting temperature. Meanwhile, NiAl displays excellent properties such as lower density and higher melting temperature than those of Ni3Al, and good thermal conductivity and oxidation resistance. These properties make it attractive for special high-temperature applications like coatings on blades in gas turbines and jet engines. However, both these alloys have the disadvantage of being quite brittle at room temperature, with Ni3Al remaining brittle at high temperatures as well. To address this problem, has been shown that Ni3Al can be made ductile when manufactured in single-crystal form rather than in polycrystalline form.

Colored gold is the name given to any gold that has been treated using techniques to change its natural color. Pure gold is slightly reddish yellow in color, but colored gold can come in a variety of different colors by alloying it with different elements.
Ti-6Al-7Nb is an alpha-beta titanium alloy first synthesized in 1977 containing 6% aluminum and 7% niobium. It features high strength and has similar properties as the cytotoxic vanadium containing alloy Ti-6Al-4V. Ti-6Al-7Nb is used as a material for hip prostheses.

Dissimilar friction stir welding (DFSW) is the application of friction stir welding (FSW), invented in The Welding Institute (TWI) in 1991, to join different base metals including aluminum, copper, steel, titanium, magnesium and other materials. It is based on solid state welding that means there is no melting. DFSW is based on a frictional heat generated by a simple tool in order to soften the materials and stir them together using both tool rotational and tool traverse movements. In the beginning, it is mainly used for joining of aluminum base metals due to existence of solidification defects in joining them by fusion welding methods such as porosity along with thick Intermetallic compounds. DFSW is taken into account as an efficient method to join dissimilar materials in the last decade. There are many advantages for DFSW in compare with other welding methods including low-cost, user-friendly, and easy operation procedure resulting in enormous usages of friction stir welding for dissimilar joints. Welding tool, base materials, backing plate (fixture), and a milling machine are required materials and equipment for DFSW. On the other hand, other welding methods, such as Shielded Metal Arc Welding (SMAW) typically need highly professional operator as well as quite expensive equipment.
References
- ↑ Fischer J (December 2000). "Mechanical, thermal, and chemical analyses of the binary system Au-Ti in the development of a dental alloy". J. Biomed. Mater. Res. 52 (4): 678–86. doi:10.1002/1097-4636(20001215)52:4<678::AID-JBM12>3.0.CO;2-P. PMID 11033550.
- ↑ Franke, P.; Neuschütz, D. (2007). "Au-Ti (Gold - Titanium)". In Franke, P.; Neuschütz, D. (eds.). Au-Ti (Gold–Titanium). Landolt-Börnstein - Group IV Physical Chemistry. Vol. 19B5. SpringerMaterials. pp. 1–5. doi:10.1007/978-3-540-45280-5_32. ISBN 978-3-540-45279-9.
- ↑ Gafner, Geoffrey (1989). "The development of 990 gold–titanium: its production, use and properties". Gold Bull. 22 (4): 112–122. doi: 10.1007/BF03214709 .
- 1 2 Svanidze, Eteri; Besara, Tiglet; Ozaydin, M. Fevsi; Tiwary, Chandra Sekhar; Wang, Jiakui K.; Radhakrishnan, Sruthi; Mani, Sendurai; Xin, Yan; Han, Ke (2016-07-01). "High hardness in the biocompatible intermetallic compound β-Ti3Au". Science Advances. 2 (7): e1600319. Bibcode:2016SciA....2E0319S. doi:10.1126/sciadv.1600319. ISSN 2375-2548. PMC 4956191 . PMID 27453942.
- ↑ "Titanium + gold = new gold standard for artificial joints". news.rice.edu. Retrieved 2016-09-17.
- ↑ "Lab discovers titanium-gold alloy that is four times harder than most steels". phys.org. Retrieved 2018-09-06.
- ↑ "Iron Man". Los Angeles Times . May 2008.
- ↑ "Iron Man | bit-tech.net".
- ↑ http://www.theinsider.com/news/842818_Robert_Downey_Jr._Says_Iron_Man_Suit_Fits_Him_Like_Gold_Titanium_Gold%5B%5D
- ↑ Pullman, Philip (3 October 2019). The Secret Commonwealth. Random House Children's Books. p. 313. ISBN 978-0553510669.