Related Research Articles

Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.
A thermoreceptor is a non-specialised sense receptor, or more accurately the receptive portion of a sensory neuron, that codes absolute and relative changes in temperature, primarily within the innocuous range. In the mammalian peripheral nervous system, warmth receptors are thought to be unmyelinated C-fibres, while those responding to cold have both C-fibers and thinly myelinated A delta fibers. The adequate stimulus for a warm receptor is warming, which results in an increase in their action potential discharge rate. Cooling results in a decrease in warm receptor discharge rate. For cold receptors their firing rate increases during cooling and decreases during warming. Some cold receptors also respond with a brief action potential discharge to high temperatures, i.e. typically above 45 °C, and this is known as a paradoxical response to heat. The mechanism responsible for this behavior has not been determined.
Transient receptor potential channels are a group of ion channels located mostly on the plasma membrane of numerous animal cell types. Most of these are grouped into two broad groups: Group 1 includes TRPC, TRPV, TRPVL, TRPM, TRPS, TRPN, and TRPA. Group 2 consists of TRPP and TRPML. Other less-well categorized TRP channels exist, including yeast channels and a number of Group 1 and Group 2 channels present in non-animals. Many of these channels mediate a variety of sensations such as pain, temperature, different kinds of tastes, pressure, and vision. In the body, some TRP channels are thought to behave like microscopic thermometers and used in animals to sense hot or cold. Some TRP channels are activated by molecules found in spices like garlic (allicin), chili pepper (capsaicin), wasabi ; others are activated by menthol, camphor, peppermint, and cooling agents; yet others are activated by molecules found in cannabis or stevia. Some act as sensors of osmotic pressure, volume, stretch, and vibration. Most of the channels are activated or inhibited by signaling lipids and contribute to a family of lipid-gated ion channels.

Melittin is the main component and the major pain-producing substance of honeybee venom. Melittin is a basic peptide consisting of 26 amino acids.

TRPV6 is a membrane calcium (Ca2+) channel protein which is particularly involved in the first step in Ca2+absorption in the intestine.

The transient receptor potential cation channel subfamily V member 1 (TRPV1), also known as the capsaicin receptor and the vanilloid receptor 1, is a protein that, in humans, is encoded by the TRPV1 gene. It was the first isolated member of the transient receptor potential vanilloid receptor proteins that in turn are a sub-family of the transient receptor potential protein group. This protein is a member of the TRPV group of transient receptor potential family of ion channels. And a receptor being clearly present in bacteria, the oldest organisms on Earth known to express phosphatidylethanolamine, the precursor to endocannabinoids, in their cytoplasmic membranes, and fatty acid metabolites with affinity for this CB receptor are produced by cyanobacteria, which diverged from eukaryotes at least 2000 million years ago (MYA).
TRPC is a family of transient receptor potential cation channels in animals.
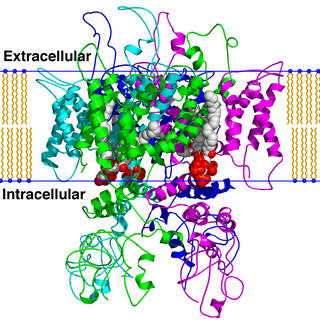
TRPV is a family of transient receptor potential cation channels in animals. All TRPVs are highly calcium selective.
TRPM is a family of transient receptor potential ion channels (M standing for wikt:melastatin). Functional TRPM channels are believed to form tetramers. The TRPM family consists of eight different channels, TRPM1–TRPM8.

Transient receptor potential canonical 1 (TRPC1) is a protein that in humans is encoded by the TRPC1 gene.

The short transient receptor potential channel 4 (TrpC4), also known as Trp-related protein 4, is a protein that in humans is encoded by the TRPC4 gene.

Transient receptor potential cation channel subfamily M member 5 (TRPM5), also known as long transient receptor potential channel 5 is a protein that in humans is encoded by the TRPM5 gene.

Transient receptor potential cation channel subfamily V member 2 is a protein that in humans is encoded by the TRPV2 gene. TRPV2 is a nonspecific cation channel that is a part of the TRP channel family. This channel allows the cell to communicate with its extracellular environment through the transfer of ions, and responds to noxious temperatures greater than 52 °C. It has a structure similar to that of potassium channels, and has similar functions throughout multiple species; recent research has also shown multiple interactions in the human body.

Transient receptor potential cation channel subfamily M (melastatin) member 8 (TRPM8), also known as the cold and menthol receptor 1 (CMR1), is a protein that in humans is encoded by the TRPM8 gene. The TRPM8 channel is the primary molecular transducer of cold somatosensation in humans. In addition, mints can desensitize a region through the activation of TRPM8 receptors.

Transient receptor potential cation channel subfamily M member 3 is a protein that in humans is encoded by the TRPM3 gene.

Transient receptor potential cation channel subfamily V member 5 is a calcium channel protein that in humans is encoded by the TRPV5 gene.
Mechanosensation is the transduction of mechanical stimuli into neural signals. Mechanosensation provides the basis for the senses of light touch, hearing, proprioception, and pain. Mechanoreceptors found in the skin, called cutaneous mechanoreceptors, are responsible for the sense of touch. Tiny cells in the inner ear, called hair cells, are responsible for hearing and balance. States of neuropathic pain, such as hyperalgesia and allodynia, are also directly related to mechanosensation. A wide array of elements are involved in the process of mechanosensation, many of which are still not fully understood.
TRPN is a member of the transient receptor potential channel family of ion channels, which is a diverse group of proteins thought to be involved in mechanoreception. The TRPN gene was given the name no mechanoreceptor potential C (nompC) when it was first discovered in fruit flies, hence the N in TRPN. Since its discovery in fruit flies, TRPN homologs have been discovered and characterized in worms, frogs, and zebrafish.
The ryanodine-inositol 1,4,5-triphosphate receptor Ca2+ channel (RIR-CaC) family includes Ryanodine receptors and Inositol trisphosphate receptors. Members of this family are large proteins, some exceeding 5000 amino acyl residues in length. This family belongs to the Voltage-gated ion channel (VIC) superfamily. Ry receptors occur primarily in muscle cell sarcoplasmic reticular (SR) membranes, and IP3 receptors occur primarily in brain cell endoplasmic reticular (ER) membranes where they effect release of Ca2+ into the cytoplasm upon activation (opening) of the channel. They are redox sensors, possibly providing a partial explanation for how they control cytoplasmic Ca2+. Ry receptors have been identified in heart mitochondria where they provide the main pathway for Ca2+ entry. Sun et al. (2011) have demonstrated oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel (RyR1;TC# 1.A.3.1.2) by NADPH oxidase 4.
The Polycystin Cation Channel (PCC) Family consists of several transporters ranging in size from 500 to over 4000 amino acyl residues (aas) in length and exhibiting between 5 and 18 transmembrane segments (TMSs). This family is a constituent of the Voltage-Gated Ion Channel (VIC) Superfamily. These transporters generally catalyze the export of cations. A representative list of proteins belonging to the PCC family can be found in the Transporter Classification Database.
References
- ↑ Vennekens R, Menigoz A, Nilius B (2012-01-01). "TRPs in the Brain". Reviews of Physiology, Biochemistry and Pharmacology. 163: 27–64. doi:10.1007/112_2012_8. ISBN 978-3-642-33520-4. PMID 23184016.
- ↑ Latorre R, Zaelzer C, Brauchi S (August 2009). "Structure-functional intimacies of transient receptor potential channels". Quarterly Reviews of Biophysics. 42 (3): 201–46. doi:10.1017/S0033583509990072. hdl: 10533/141344 . PMID 20025796. S2CID 24518599.
- 1 2 Montell C (February 2005). "The TRP superfamily of cation channels". Science's STKE. 2005 (272): re3. doi:10.1126/stke.2722005re3. PMID 15728426. S2CID 7326120.
- ↑ Ramsey IS, Delling M, Clapham DE (2006-01-01). "An introduction to TRP channels". Annual Review of Physiology. 68: 619–47. doi:10.1146/annurev.physiol.68.040204.100431. PMID 16460286.
- ↑ Clapham DE (April 2007). "SnapShot: mammalian TRP channels". Cell. 129 (1): 220.e1–220.e2. doi: 10.1016/j.cell.2007.03.034 . PMID 17418797. S2CID 597250.
- ↑ Feng Z, Li W, Ward A, Piggott BJ, Larkspur ER, Sternberg PW, Xu XZ (November 2006). "A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels". Cell. 127 (3): 621–33. doi:10.1016/j.cell.2006.09.035. PMC 2859215 . PMID 17081982.
- ↑ Runnels LW, Yue L, Clapham DE (February 2001). "TRP-PLIK, a bifunctional protein with kinase and ion channel activities". Science. 291 (5506): 1043–7. Bibcode:2001Sci...291.1043R. doi:10.1126/science.1058519. PMID 11161216. S2CID 30327400.
- ↑ Liu L, Li Y, Wang R, Yin C, Dong Q, Hing H, Kim C, Welsh MJ (November 2007). "Drosophila hygrosensation requires the TRP channels water witch and nanchung". Nature. 450 (7167): 294–8. Bibcode:2007Natur.450..294L. doi:10.1038/nature06223. PMID 17994098. S2CID 4426557.
- 1 2 Binshtok AM, Bean BP, Woolf CJ (October 2007). "Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers". Nature. 449 (7162): 607–10. Bibcode:2007Natur.449..607B. doi:10.1038/nature06191. PMID 17914397. S2CID 6374938.
- ↑ Mio K, Ogura T, Sato C (May 2008). "Structure of six-transmembrane cation channels revealed by single-particle analysis from electron microscopic images". Journal of Synchrotron Radiation. 15 (Pt 3): 211–4. doi:10.1107/S0909049508004640. PMC 2394823 . PMID 18421141.
- ↑ Dodier Y, Banderali U, Klein H, Topalak O, Dafi O, Simoes M, Bernatchez G, Sauvé R, Parent L (February 2004). "Outer pore topology of the ECaC-TRPV5 channel by cysteine scan mutagenesis". The Journal of Biological Chemistry. 279 (8): 6853–62. doi: 10.1074/jbc.M310534200 . PMID 14630907.
- ↑ Dohke Y, Oh YS, Ambudkar IS, Turner RJ (March 2004). "Biogenesis and topology of the transient receptor potential Ca2+ channel TRPC1". The Journal of Biological Chemistry. 279 (13): 12242–8. doi: 10.1074/jbc.M312456200 . PMID 14707123.
- ↑ Hardie RC, Minke B (September 1993). "Novel Ca2+ channels underlying transduction in Drosophila photoreceptors: implications for phosphoinositide-mediated Ca2+ mobilization". Trends in Neurosciences. 16 (9): 371–6. doi:10.1016/0166-2236(93)90095-4. PMID 7694408. S2CID 3971401.
- ↑ Phelps CB, Huang RJ, Lishko PV, Wang RR, Gaudet R (February 2008). "Structural analyses of the ankyrin repeat domain of TRPV6 and related TRPV ion channels". Biochemistry. 47 (8): 2476–84. doi:10.1021/bi702109w. PMC 3006163 . PMID 18232717.
- ↑ Brauchi S, Orio P (2011-01-01). "Voltage Sensing in Thermo-TRP Channels". Transient Receptor Potential Channels. Advances in Experimental Medicine and Biology. Vol. 704. pp. 517–30. doi:10.1007/978-94-007-0265-3_28. ISBN 978-94-007-0264-6. PMID 21290314.
- 1 2 Hu, Hongzhen; Bandell, Michael; Grandl, Jorg; Petrus, Matt (2011-01-01). Zhu, Michael X. (ed.). High-Throughput Approaches to Studying Mechanisms of TRP Channel Activation. Boca Raton (FL): CRC Press/Taylor & Francis. ISBN 9781439818602. PMID 22593966.
As of this edit, this article uses content from "1.A.4 The Transient Receptor Potential Ca2+ Channel (TRP-CC) Family" , which is licensed in a way that permits reuse under the Creative Commons Attribution-ShareAlike 3.0 Unported License, but not under the GFDL. All relevant terms must be followed.