Related Research Articles

The kidneys are two reddish-brown bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and in adult humans are about 12 centimetres in length. They receive blood from the paired renal arteries; blood exits into the paired renal veins. Each kidney is attached to a ureter, a tube that carries excreted urine to the bladder.
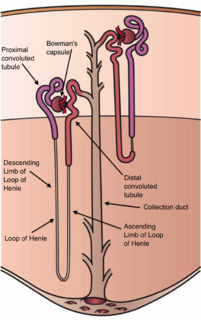
The nephron is the minute or microscopic structural and functional unit of the kidney. It is composed of a renal corpuscle and a renal tubule. The renal corpuscle consists of a tuft of capillaries called a glomerulus and a cup-shaped structure called Bowman's capsule. The renal tubule extends from the capsule. The capsule and tubule are connected and are composed of epithelial cells with a lumen. A healthy adult has 1 to 1.5 million nephrons in each kidney. Blood is filtered as it passes through three layers: the endothelial cells of the capillary wall, its basement membrane, and between the foot processes of the podocytes of the lining of the capsule. The tubule has adjacent peritubular capillaries that run between the descending and ascending portions of the tubule. As the fluid from the capsule flows down into the tubule, it is processed by the epithelial cells lining the tubule: water is reabsorbed and substances are exchanged ; first with the interstitial fluid outside the tubules, and then into the plasma in the adjacent peritubular capillaries through the endothelial cells lining that capillary. This process regulates the volume of body fluid as well as levels of many body substances. At the end of the tubule, the remaining fluid—urine—exits: it is composed of water, metabolic waste, and toxins.

The collecting duct system of the kidney consists of a series of tubules and ducts that physically connect nephrons to a minor calyx or directly to the renal pelvis. The collecting duct system is the last part of nephron and participates in electrolyte and fluid balance through reabsorption and excretion, processes regulated by the hormones aldosterone and vasopressin.

Renal functions include maintaining an acid–base balance; regulating fluid balance; regulating sodium, potassium, and other electrolytes; clearing toxins; absorption of glucose, amino acids, and other small molecules; regulation of blood pressure; production of various hormones, such as erythropoietin; and activation of vitamin D.

Renal physiology is the study of the physiology of the kidney. This encompasses all functions of the kidney, including maintenance of acid-base balance; regulation of fluid balance; regulation of sodium, potassium, and other electrolytes; clearance of toxins; absorption of glucose, amino acids, and other small molecules; regulation of blood pressure; production of various hormones, such as erythropoietin; and activation of vitamin D.

The proximal tubule is the segment of the nephron in kidneys which begins from the renal pole of the Bowman's capsule to the beginning of loop of Henle. It can be further classified into the proximal convoluted tubule (PCT) and the proximal straight tubule (PST).

In the kidney, the loop of Henle is the portion of a nephron that leads from the proximal convoluted tubule to the distal convoluted tubule. Named after its discoverer, the German anatomist Friedrich Gustav Jakob Henle, the loop of Henle's main function is to create a concentration gradient in the medulla of the kidney.

Assessment of kidney function occurs in different ways, using the presence of symptoms and signs, as well as measurements using urine tests, blood tests, and medical imaging.
In physiology, the renal threshold is the concentration of a substance dissolved in the blood above which the kidneys begin to remove it into the urine. When the renal threshold of a substance is exceeded, reabsorption of the substance by the proximal convoluted tubule is incomplete; consequently, part of the substance remains in the urine. Renal thresholds vary by substance – the low potency poison urea, for instance, is removed at much lower concentrations than glucose. Indeed, the most common reason for the glucose renal threshold ever being exceeded is diabetes, which is called glycosuria.

Loop diuretics are diuretics that act on the Na-K-Cl cotransporter along the thick ascending limb of the loop of Henle in the kidney. They are primarily used in medicine to treat hypertension and edema often due to congestive heart failure or chronic kidney disease. While thiazide diuretics are more effective in patients with normal kidney function, loop diuretics are more effective in patients with impaired kidney function.

Glycosuria is the excretion of glucose into the urine. Ordinarily, urine contains no glucose because the kidneys are able to reabsorb all of the filtered glucose from the tubular fluid back into the bloodstream. Glycosuria is nearly always caused by elevated blood glucose levels, most commonly due to untreated diabetes mellitus. Rarely, glycosuria is due to an intrinsic problem with glucose reabsorption within the kidneys, producing a condition termed renal glycosuria. Glycosuria leads to excessive water loss into the urine with resultant dehydration, a process called osmotic diuresis.
In pharmacology, clearance is a pharmacokinetic measurement of the volume of plasma from which a substance is completely removed per unit time. Usually, clearance is measured in L/h or mL/min. The quantity reflects the rate of drug elimination divided by plasma concentration. Excretion, on the other hand, is a measurement of the amount of a substance removed from the body per unit time. While clearance and excretion of a substance are related, they are not the same thing. The concept of clearance was described by Thomas Addis, a graduate of the University of Edinburgh Medical School.

In the renal system, peritubular capillaries are tiny blood vessels, supplied by the efferent arteriole, that travel alongside nephrons allowing reabsorption and secretion between blood and the inner lumen of the nephron. Peritubular capillaries surround the cortical parts of the proximal and distal tubules, while the vasa recta go into the medulla to approach the loop of Henle.
Sodium-dependent glucose cotransporters are a family of glucose transporter found in the intestinal mucosa (enterocytes) of the small intestine (SGLT1) and the proximal tubule of the nephron. They contribute to renal glucose reabsorption. In the kidneys, 100% of the filtered glucose in the glomerulus has to be reabsorbed along the nephron. If the plasma glucose concentration is too high (hyperglycemia), glucose passes into the urine (glucosuria) because SGLT are saturated with the filtered glucose.
Renal reabsorption of sodium (Na+) is a part of renal physiology. It uses Na-H antiport, Na-glucose symport, sodium ion channels (minor). It is stimulated by angiotensin II and aldosterone, and inhibited by atrial natriuretic peptide.
Renal glucose reabsorption is the part of kidney (renal) physiology that deals with the retrieval of filtered glucose, preventing it from disappearing from the body through the urine.
In physiology, splay is the difference between urine threshold and saturation, or TM, where saturation is the exhausted supply of renal reabsorption carriers. In simpler terms, splay is the concentration difference between a substance's maximum renal reabsorption vs. appearance in the urine. Splay is usually used in reference to glucose; other substances, such as phosphate, have virtually no splay at all. Splay appears to occur because kidney nephrons do not have the same tubular maximum for glucose (TmG) therefore some nephrons may excrete before others and also because "the maximum reabsorption rate cannot be achieved until the amount/min of glucose being presented to the renal tubules is great enough to fully saturate the receptor sites". John Field of the American Physiological Society said "Since the splay may occur when the residual nephrons are said to be free of anatomic abnormalities, the possibility exists that changes in the kinetics of glucose reabsorption may have been induced".

In pharmacology the elimination or excretion of a drug is understood to be any one of a number of processes by which a drug is eliminated from an organism either in an unaltered form or modified as a metabolite. The kidney is the main excretory organ although others exist such as the liver, the skin, the lungs or glandular structures, such as the salivary glands and the lacrimal glands. These organs or structures use specific routes to expel a drug from the body, these are termed elimination pathways:
Gliflozins are a class of drugs in the treatment of type 2 diabetes (T2D). They act by inhibiting sodium/glucose cotransporter 2 (SGLT-2), and are therefore also called SGLT-2 inhibitors. The efficacy of the drug is dependent on renal excretion and prevents glucose from going into blood circulation by promoting glucosuria. The mechanism of action is insulin independent.
The rock dove, Columbia livia, has a number of special adaptations for regulating water uptake and loss.
References
- ↑ DeFronzo, Ralph A.; Hompesch, Marcus; Kasichayanula, Sreeneeranj; Liu, Xiaoni; Hong, Ying; Pfister, Marc; Morrow, Linda A.; Leslie, Bruce R.; Boulton, David W. (October 2013). "Characterization of Renal Glucose Reabsorption in Response to Dapagliflozin in Healthy Subjects and Subjects With Type 2 Diabetes". Diabetes Care. 36 (10): 3169–3176. doi:10.2337/dc13-0387. ISSN 0149-5992. PMC 3781504 . PMID 23735727.
- ↑ Sect. 7, Ch. 5: Renal Threshold Archived 2006-09-01 at the Wayback Machine
- ↑ Tubular Transport