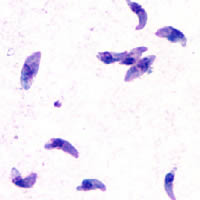
Toxoplasmosis is a parasitic disease caused by Toxoplasma gondii, an apicomplexan. Infections with toxoplasmosis are associated with a variety of neuropsychiatric and behavioral conditions. Occasionally, people may have a few weeks or months of mild, flu-like illness such as muscle aches and tender lymph nodes. In a small number of people, eye problems may develop. In those with a weak immune system, severe symptoms such as seizures and poor coordination may occur. If a woman becomes infected during pregnancy, a condition known as congenital toxoplasmosis may affect the child.

Trichinosis, also known as trichinellosis, is a parasitic disease caused by roundworms of the Trichinella type. During the initial infection, invasion of the intestines can result in diarrhea, abdominal pain, and vomiting. Migration of larvae to muscle, which occurs about a week after being infected, can cause swelling of the face, inflammation of the whites of the eyes, fever, muscle pains, and a rash. Minor infection may be without symptoms. Complications may include inflammation of heart muscle, central nervous system involvement, and inflammation of the lungs.

Toxoplasma gondii is a parasitic protozoan that causes toxoplasmosis. Found worldwide, T. gondii is capable of infecting virtually all warm-blooded animals, but felids are the only known definitive hosts in which the parasite may undergo sexual reproduction.

Parasitology is the study of parasites, their hosts, and the relationship between them. As a biological discipline, the scope of parasitology is not determined by the organism or environment in question but by their way of life. This means it forms a synthesis of other disciplines, and draws on techniques from fields such as cell biology, bioinformatics, biochemistry, molecular biology, immunology, genetics, evolution and ecology.
Ingestion is the consumption of a substance by an organism. In animals, it normally is accomplished by taking in a substance through the mouth into the gastrointestinal tract, such as through eating or drinking. In single-celled organisms, ingestion takes place by absorbing a substance through the cell membrane.
Toxocariasis is an illness of humans caused by the dog roundworm and, less frequently, the cat roundworm. These are the most common intestinal roundworms of dogs, coyotes, wolves and foxes and domestic cats, respectively. Humans are among the many "accidental" or paratenic hosts of these roundworms.
Gnathostomiasis, also known as larva migrans profundus, is the human infection caused by the nematode Gnathostoma spinigerum and/or Gnathostoma hispidum, which infects vertebrates.

Anisakis is a genus of parasitic nematodes that have life cycles involving fish and marine mammals. They are infective to humans and cause anisakiasis. People who produce immunoglobulin E in response to this parasite may subsequently have an allergic reaction, including anaphylaxis, after eating fish infected with Anisakis species.

Trichinella spiralis is a viviparous nematode parasite, occurring in rodents, pigs, bears, hyenas and humans, and is responsible for the disease trichinosis. It is sometimes referred to as the "pork worm" due to it being typically encountered in undercooked pork products. It should not be confused with the distantly related pork tapeworm.

Trichinella is the genus of parasitic roundworms of the phylum Nematoda that cause trichinosis. Members of this genus are often called trichinella or trichina worms. A characteristic of Nematoda is the one-way digestive tract, with a pseudocoelom.

Paragonimus is a genus of flukes (trematodes) and is the only genus in the monotypic family Paragonimidae. Some tens of species have been described, but they are difficult to distinguish, so it is not clear how many of the named species may be synonyms. The name Paragonimus is derived from the combination of two Greek words, “para” and “gonimos”. Several of the species are known as lung flukes. In humans some of the species occur as zoonoses; the term for the condition is paragonimiasis. The first intermediate hosts of Paragonimus include at least 54 species of freshwater snails from superfamilies Cerithioidea and Rissooidea.
Spirometra erinaceieuropaei is a parasitic tapeworm that infects domestic animals and humans. The medical term for this infection in humans and other animals is sparganosis. Morphologically, these worms are similar to other worms in the genus Spirometra. They have a long body consisting of three sections: the scolex, the neck, and the strobilia. They have a complex life cycle that consists of three hosts, and can live in varying environments and bodily tissues. Humans can contract this parasite in three main ways. Historically, humans are considered a paratenic host; however, the first case of an adult S. erinaceieuropaei infection in humans was reported in 2017. Spirometra tapeworms exist worldwide and infection is common in animals, but S. erinaceieuropaei infections are rare in humans. Treatment for infection typically includes surgical removal and anti-worm medication.

Toxocara canis is a worldwide-distributed helminth parasite that primarily infects dogs and other canids, but can also infect other animals including humans. The name is derived from the Greek word "toxon," meaning bow or quiver, and the Latin word "caro," meaning flesh. T. canis live in the small intestine of the definitive host. This parasite is very common in puppies and somewhat less common in adult dogs. In adult dogs, infection is usually asymptomatic but may be characterized by diarrhea. By contrast, untreated infection with Toxocara canis can be fatal in puppies, causing diarrhea, vomiting, pneumonia, enlarged abdomen, flatulence, poor growth rate, and other complications.

Capillaria hepatica is a parasitic nematode which causes hepatic capillariasis in rodents and numerous other mammal species, including humans. The life cycle of C. hepatica may be completed in a single host species. However, the eggs, which are laid in the liver, must mature outside of the host body prior to infecting a new host. So the death of the host in which the adults reach sexual maturity, either by being eaten or dying and decomposing, is necessary for completion of the life cycle.

Heterophyes heterophyes, or the intestinal fish fluke, was discovered by Theodor Maximaillian Bilharz in 1851. This parasite was found during an autopsy of an Egyptian mummy. H. heterophyes is found in the Middle East, West Europe and Africa. They use different species to complete their complex lifestyle. Humans and other mammals are the definitive host, first intermediate host are snails, and second intermediate are fish. Mammals that come in contact with the parasite are dogs, humans, and cats. Snails that are affected by this parasite are the Cerithideopsilla conica. Fish that come in contact with this parasite are Mugil cephalus, Tilapia milotica, Aphanius fasciatus, and Acanthgobius sp. Humans and mammals will come in contact with this parasite by the consumption of contaminated or raw fish. This parasite is one of the smallest endoparasite to infect humans. It can cause intestinal infection called heterophyiasis.
Trichinella nativa is a nematode worm, one of the species of the genus Trichinella, found in arctic and subarctic regions.

Alaria is a genus of flatworms, or trematodes, in the family Diplostomidae.
Trichinella papuae is a nematode parasite responsible for a zoonotic disease called trichinellosis, predominantly in Thailand. Currently, eight species of Trichinella are known.

Pseudoterranova is a genus within the family Anisakidae of parasitic nematodes with an aquatic life cycle. The lifecycle of Pseudoterranova spp. involves marine mammals, pinnipeds as definitive hosts, planktonic or benthic crustaceans as intermediate hosts and fish which act as second intermediate or paratenic hosts. In some regions, the rise in seal numbers has prefaced a significant increase in fish infected with P. decipiens which is of concern for fish health. Infection with Pseudoterranova may affect the health and swimming ability of the fish host and is therefore of concern for the survival of wild caught and farmed species. Species belonging to this genus have been demonstrated to cause illness of varying exigency in humans if raw or under cooked infected fish is consumed. Cases of human infection have been reported from consuming partially cooked fish infected with Pseudoterranova decipiens, Pseudoterranova cattani and Pseudoterranova azarasi. The propensity of P. decipiens to encyst in the edible portion of fish musculature may make this parasite a considerable threat to human health in undercooked fish.
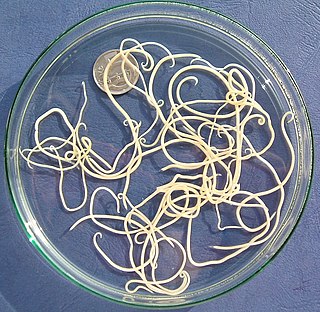
Nematode infection in dogs - the infection of dogs with parasitic nemamotodes - are, along with tapeworm infections and infections with protozoa, frequent parasitoses in veterinary practice. Nematodes, as so-called endoparasites, colonize various internal organs - most of them the digestive tract - and the skin. To date, about 30 different species of nematode have been identified in domestic dogs; they are essentially also found in wild dog species. However, the majority of them often cause no or only minor symptoms of disease in adult animals. The infection therefore does not necessarily have to manifest itself in a worm disease (helminthosis). For most nematodes, an infection can be detected by examining the feces for eggs or larvae. Roundworm infection in dogs and the hookworm in dogs is of particular health significance in Central Europe, as they can also be transmitted to humans (zoonosis). Regular deworming can significantly reduce the frequency of infection and thus the risk of infection for humans and dogs.











