
An endosymbiont or endobiont is an organism that lives within the body or cells of another organism. Typically the two organisms are in a mutualistic relationship. Examples are nitrogen-fixing bacteria, which live in the root nodules of legumes, single-cell algae inside reef-building corals and bacterial endosymbionts that provide essential nutrients to insects.
The evolution of flagella is of great interest to biologists because the three known varieties of flagella – each represent a sophisticated cellular structure that requires the interaction of many different systems.

Riftia pachyptila, commonly known as the giant tube worm and less commonly known as the giant beardworm, is a marine invertebrate in the phylum Annelida related to tube worms commonly found in the intertidal and pelagic zones. R. pachyptila lives on the floor of the Pacific Ocean near hydrothermal vents. The vents provide a natural ambient temperature in their environment ranging from 2 to 30 °C, and this organism can tolerate extremely high hydrogen sulfide levels. These worms can reach a length of 3 m, and their tubular bodies have a diameter of 4 cm (1.6 in).

The parabasalids are a group of flagellated protists within the supergroup Excavata. Most of these eukaryotic organisms form a symbiotic relationship in animals. These include a variety of forms found in the intestines of termites and cockroaches, many of which have symbiotic bacteria that help them digest cellulose in woody plants. Other species within this supergroup are known parasites, and include human pathogens.
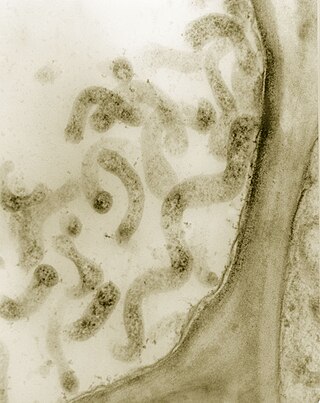
Spiroplasma is a genus of Mollicutes, a group of small bacteria without cell walls. Spiroplasma shares the simple metabolism, parasitic lifestyle, fried-egg colony morphology and small genome of other Mollicutes, but has a distinctive helical morphology, unlike Mycoplasma. It has a spiral shape and moves in a corkscrew motion. Many Spiroplasma are found either in the gut or haemolymph of insects where they can act to manipulate host reproduction, or defend the host as endosymbionts. Spiroplasma are also disease-causing agents in the phloem of plants. Spiroplasmas are fastidious organisms, which require a rich culture medium. Typically they grow well at 30 °C, but not at 37 °C. A few species, notably Spiroplasma mirum, grow well at 37 °C, and cause cataracts and neurological damage in suckling mice. The best studied species of spiroplasmas are Spiroplasma poulsonii, a reproductive manipulator and defensive insect symbiont, Spiroplasma citri, the causative agent of citrus stubborn disease, and Spiroplasma kunkelii, the causative agent of corn stunt disease.

Reticulitermes flavipes, the eastern subterranean termite, is the most common termite found in North America. These termites are the most economically important wood destroying insects in the United States and are classified as pests. They feed on cellulose material such as the structural wood in buildings, wooden fixtures, paper, books, and cotton. A mature colony can range from 20,000 workers to as high as 5 million workers and the primary queen of the colony lays 5,000 to 10,000 eggs per year to add to this total.
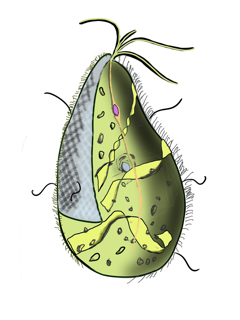
Mixotricha paradoxa is a species of protozoan that lives inside the gut of the Australian termite species Mastotermes darwiniensis.
Symbiotic bacteria are bacteria living in symbiosis with another organism or each other. For example, rhizobia living in root nodules of legumes provide nitrogen fixing activity for these plants.
Neocallimastigomycota is a phylum containing anaerobic fungi, which are symbionts found in the digestive tracts of larger herbivores. Anaerobic fungi were originally placed within phylum Chytridiomycota, within Order Neocallimastigales but later raised to phylum level, a decision upheld by later phylogenetic reconstructions. It encompasses only one family.
Blattabacterium is a genus of obligate mutualistic endosymbiont bacteria that are believed to inhabit all species of cockroach studied to date, with the exception of the genus Nocticola. The genus' presence in the termite Mastotermes darwiniensis led to speculation, later confirmed, that termites and cockroaches are evolutionarily linked.
Sodalis is a genus of bacteria within the family Pectobacteriaceae. This genus contains several insect endosymbionts and also a free-living group. It is studied due to its potential use in the biological control of the tsetse fly. Sodalis is an important model for evolutionary biologists because of its nascent endosymbiosis with insects.

Paracatenula is a genus of millimeter sized free-living marine gutless catenulid flatworms.
Microbial symbiosis in marine animals was not discovered until 1981. In the time following, symbiotic relationships between marine invertebrates and chemoautotrophic bacteria have been found in a variety of ecosystems, ranging from shallow coastal waters to deep-sea hydrothermal vents. Symbiosis is a way for marine organisms to find creative ways to survive in a very dynamic environment. They are different in relation to how dependent the organisms are on each other or how they are associated. It is also considered a selective force behind evolution in some scientific aspects. The symbiotic relationships of organisms has the ability to change behavior, morphology and metabolic pathways. With increased recognition and research, new terminology also arises, such as holobiont, which the relationship between a host and its symbionts as one grouping. Many scientists will look at the hologenome, which is the combined genetic information of the host and its symbionts. These terms are more commonly used to describe microbial symbionts.
Stilbonematinae is a subfamily of the nematode worm family Desmodoridae that is notable for its symbiosis with sulfur-oxidizing bacteria.

Cryptocercus punctulatus, known generally as brown-hooded cockroach, is a species of cockroach in the family Cryptocercidae. Other common names include the woodroach, wingless wood roach, and eastern wood-eating cockroach. It is found in North America.
Renate Radek is a protistologist and an associate professor at the Freie Universität Berlin.
Saccinobaculus is a genus of unicellular eukaryotes that resides in the hindgut of the wood-feeding cockroach Cryptocercus punctulatus. This genus is known for its distinctive movement that resembles a snake trashing in a bag. The genus is involved in the digestion of wood materials within its insect-host and is vertically transmitted to insect progeny. The genus is the part of the family Saccinobaculidae.
Novymonas esmeraldas is a protist and member of flagellated trypanosomatids. It is an obligate parasite in the gastrointestinal tract of a bug, and is in turn a host to symbiotic bacteria. It maintains strict mutualistic relationship with the bacteria as a sort of cell organelle (endosymbiont) so that it cannot lead an independent life without the bacteria. Its discovery in 2016 suggests that it is a good model in the evolution of prokaryotes into eukaryotes by symbiogenesis. The endosymbiotic bacterium was identified as member of the genus Pandoraea.
Holomastigotoides is a genus of parabasalids found in the hindgut of lower termites. It is characterized by its dense, organized arrangement of flagella on the cell surface and the presence of a mitotic spindle outside its nucleus during the majority of its cell cycle. As a symbiont of termites, Holomastigotoides is able to ingest wood and aid its host in digestion. In return, Holomastigotoides is supplied with a stable habitat and steady supply of food. Holomastigotoides has notably been studied to observe the mechanisms of chromosomal pairing and segregation in haploid and diploid cells.
Monocercomonas is a Parabasalian genus belonging to the order Trichomonadida. It presents four flagella, three forward-facing and one trailing, without the presence of a costa or any kind of undulating membrane. Monocercomonas is found in animal guts. and is susceptible to cause Monocercomoniasis in reptiles








