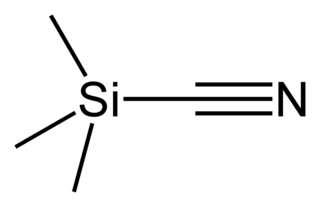In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

In organic chemistry, a cyanohydrin or hydroxynitrile is a functional group found in organic compounds in which a cyano and a hydroxy group are attached to the same carbon atom. The general formula is R2C(OH)CN, where R is H, alkyl, or aryl. Cyanohydrins are industrially important precursors to carboxylic acids and some amino acids. Cyanohydrins can be formed by the cyanohydrin reaction, which involves treating a ketone or an aldehyde with hydrogen cyanide (HCN) in the presence of excess amounts of sodium cyanide (NaCN) as a catalyst:

Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost universally used as a solution in diethyl ether. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions. Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent trimethylsilyldiazomethane.
In organic chemistry, the diazo group is an organic moiety consisting of two linked nitrogen atoms at the terminal position. Overall charge-neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds or diazoalkanes and are described by the general structural formula R2C=N+=N−. The simplest example of a diazo compound is diazomethane, CH2N2. Diazo compounds should not be confused with azo compounds or with diazonium compounds.

A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si(CH3)3], which is in turn bonded to the rest of a molecule. This structural group is characterized by chemical inertness and a large molecular volume, which makes it useful in a number of applications.
In organic chemistry, the Arndt–Eistert reaction is the conversion of a carboxylic acid to its homologue. Named for the German chemists Fritz Arndt (1885–1969) and Bernd Eistert (1902–1978), the method entails treating an acid chlorides with diazomethane. It is a popular method of producing β-amino acids from α-amino acids.

The Favorskii rearrangement is principally a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid, but usually either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α'-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds.

Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound, with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry.

Organosilicon chemistry is the study of organometallic compounds containing carbon–silicon bonds, to which they are called organosilicon compounds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.
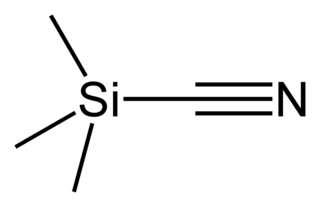
Trimethylsilyl cyanide is the chemical compound with the formula (CH3)3SiCN. This volatile liquid consists of a cyanide group, that is CN, attached to a trimethylsilyl group. The molecule is used in organic synthesis as the equivalent of hydrogen cyanide. It is prepared by the reaction of lithium cyanide and trimethylsilyl chloride:

Lead(IV) acetate or lead tetraacetate is an metalorganic compound with chemical formula Pb(C2H3O2)4. It is a colorless solid that is soluble in nonpolar, organic solvents, indicating that it is not a salt. It is degraded by moisture and is typically stored with additional acetic acid. The compound is used in organic synthesis.
Bis(trimethylsilyl)amine (also known as hexamethyldisilazane and HMDS) is an organosilicon compound with the molecular formula [(CH3)3Si]2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms. An electron diffraction study shows that silicon-nitrogen bond length (173.5 pm) and Si-N-Si bond angle (125.5°) to be similar to disilazane (in which methyl groups are replaced by hydrogen atoms) suggesting that steric factors are not a factor in regulating angles in this case. This colorless liquid is a reagent and a precursor to bases that are popular in organic synthesis and organometallic chemistry. Additionally, HMDS is also increasingly used as molecular precursor in chemical vapor deposition techniques to deposit silicon carbonitride thin films or coatings.

Trimethylsilyl azide is the organosilicon compound with the formula (CH3)3SiN3. A colorless liquid, it is a reagent in organic chemistry, serving as the equivalent of hydrazoic acid.
Acetone cyanohydrin (ACH) is an organic compound used in the production of methyl methacrylate, the monomer of the transparent plastic polymethyl methacrylate (PMMA), also known as acrylic. It liberates hydrogen cyanide easily, so it is used as a source of such. For this reason, this cyanohydrin is also highly toxic.

Trimethylsilyl trifluoromethanesulfonate (TMSOTf) is an organosilicon compound with the formula (CH3)3SiO3SCF3. It is a colorless moisture-sensitive liquid. It is the trifluoromethanesulfonate derivative of trimethylsilyl. It is mainly used to activate ketones and aldehydes in organic synthesis.

In organic chemistry, carbonyl reduction is the conversion of any carbonyl group, usually to an alcohol. It is a common transformation that is practiced in many ways. Ketones, aldehydes, carboxylic acids, esters, amides, and acid halides - some of the most pervasive functional groups, -comprise carbonyl compounds. Carboxylic acids, esters, and acid halides can be reduced to either aldehydes or a step further to primary alcohols, depending on the strength of the reducing agent. Aldehydes and ketones can be reduced respectively to primary and secondary alcohols. In deoxygenation, the alcohol group can be further reduced and removed altogether by replacement with H.
An insertion reaction is a chemical reaction where one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:

Trimethylsilyl iodide (iodotrimethylsilane or TMSI) is an organosilicon compound with the chemical formula (CH3)3SiI. It is a colorless, volatile liquid at room temperature.

(Trimethylsilyl)methyllithium is classified both as an organolithium compound and an organosilicon compound. It has the empirical formula LiCH2Si(CH3)3, often abbreviated LiCH2tms. It crystallizes as the hexagonal prismatic hexamer [LiCH2tms]6, akin to some polymorphs of methyllithium. Many adducts have been characterized including the diethyl ether complexed cubane [Li4(μ3-CH2tms)4(Et2O)2] and [Li2(μ-CH2tms)2(tmeda)2].

(Trimethylsilyl)methyl chloride is the organosilicon compound with the formula (CH3)3SiCH2Cl. A colorless, volatile liquid, it is an alkylating agent that is employed in organic synthesis, especially as a precursor to (trimethylsilyl)methyllithium. In the presence of triphenylphosphine, it olefinates benzophenones: