
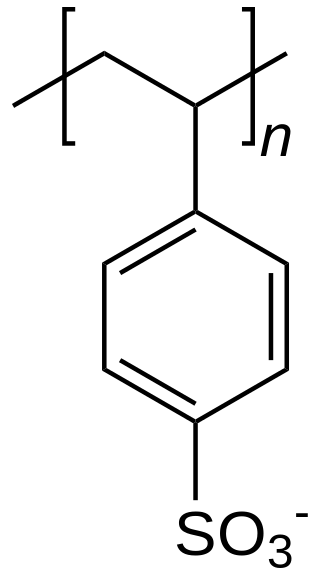
The haloalkanes are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

In chemistry, the term phosphonium describes polyatomic cations with the chemical formula PR+
4. These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.

Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides/oxychlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride.

Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride.

Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.
In chemistry, there are three series of binary phosphorus halides, containing phosphorus in the oxidation states +5, +3 and +2. All compounds have been described, in varying degrees of detail, although serious doubts have been cast on the existence of PI5. Mixed chalcogen halides also exist.
In chemistry, a counterion is the ion that accompanies an ionic species in order to maintain electric neutrality. In table salt the sodium ion is the counterion for the chloride ion and vice versa.

Tantalum(V) chloride, also known as tantalum pentachloride, is an inorganic compound with the formula TaCl5. It takes the form of a white powder and is commonly used as a starting material in tantalum chemistry. It readily hydrolyzes to form tantalum(V) oxychloride (TaOCl3) and eventually tantalum pentoxide (Ta2O5); this requires that it be synthesised and manipulated under anhydrous conditions, using air-free techniques.

A Grignard reagent or Grignard compound is a chemical compound with the general formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH3 and phenylmagnesium bromide (C6H5)−Mg−Br. They are a subclass of the organomagnesium compounds.

Molybdenum(V) chloride is the inorganic compound with the empirical formula MoCl5. This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents.

Indium(III) chloride is the chemical compound with the formula InCl3. This salt is a white, flaky solid with applications in organic synthesis as a Lewis acid. It is also the most available soluble derivative of indium. This is one of three known indium chlorides.

Methyllithium is the simplest organolithium reagent with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used in solution with an ether as the solvent, is a reagent in organic synthesis as well as organometallic chemistry. Operations involving methyllithium require anhydrous conditions, because the compound is highly reactive toward water. Oxygen and carbon dioxide are also incompatible with MeLi. Methyllithium is usually not prepared, but purchased as a solution in various ethers.

Triphenylphosphine oxide (often abbreviated TPPO) is the organophosphorus compound with the formula OP(C6H5)3, also written as Ph3PO or PPh3O (Ph = C6H5). This colourless crystalline compound is a common but potentially useful waste product in reactions involving triphenylphosphine. It is a popular reagent to induce the crystallizing of chemical compounds.

Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis, and reactions. Organotitanium compounds in organometallic chemistry contain carbon-titanium chemical bonds. They are reagents in organic chemistry and are involved in major industrial processes.

Bis(triphenylphosphine)iminium chloride is the chemical compound with the formula [( 3P)2N]Cl, often abbreviated [(Ph3P)2N]Cl, where Ph is phenyl C6H5, or even abbreviated [PPN]Cl or [PNP]Cl or PPNCl or PNPCl, where PPN or PNP stands for (Ph3P)2N. This colorless salt is a source of the [(Ph3P)2N]+ cation, which is used as an unreactive and weakly coordinating cation to isolate reactive anions. [(Ph3P)2N]+ is a phosphazene.
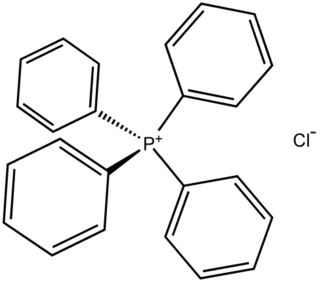
Tetraphenylphosphonium chloride is the chemical compound with the formula (C6H5)4PCl, abbreviated Ph4PCl or PPh4Cl. Tetraphenylphosphonium and especially tetraphenylarsonium salts were formerly of interest in gravimetric analysis of perchlorate and related oxyanions. This colourless salt is used to generate lipophilic salts from inorganic and organometallic anions. Thus, Ph4P+ is useful as a phase-transfer catalyst, again because it allows inorganic anions to dissolve in organic solvents.
Organomanganese chemistry is the chemistry of organometallic compounds containing a carbon to manganese chemical bond. In a 2009 review, Cahiez et al. argued that as manganese is cheap and benign, organomanganese compounds have potential as chemical reagents, although currently they are not widely used as such despite extensive research.
In organic chemistry, thiocarboxylic acids or carbothioic acids are organosulfur compounds related to carboxylic acids by replacement of one of the oxygen atoms with a sulfur atom. Two tautomers are possible: a thione form and a thiol form. These are sometimes also referred to as "carbothioic O-acid" and "carbothioic S-acid" respectively. Of these the thiol form is most common.

(2-Bromophenyl)diphenylphosphine is an organophosphorus compound with the formula (C6H4Br)P(C6H5)2. It is a white crystalline solid that is soluble in nonpolar organic solvents. The compound is used as a precursor to the 2-lithiated derivative of triphenylphosphine, which in turn is a precursor to other phosphine ligands.





















