
The ozone layer or ozone shield is a region of Earth's stratosphere that absorbs most of the Sun's ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the atmosphere, although still small in relation to other gases in the stratosphere. The ozone layer contains less than 10 parts per million of ozone, while the average ozone concentration in Earth's atmosphere as a whole is about 0.3 parts per million. The ozone layer is mainly found in the lower portion of the stratosphere, from approximately 15 to 35 kilometers (9 to 22 mi) above Earth, although its thickness varies seasonally and geographically.

Ozone depletion consists of two related events observed since the late 1970s: a steady lowering of about four percent in the total amount of ozone in Earth's atmosphere, and a much larger springtime decrease in stratospheric ozone around Earth's polar regions. The latter phenomenon is referred to as the ozone hole. There are also springtime polar tropospheric ozone depletion events in addition to these stratospheric events.

The stratosphere is the second layer of the atmosphere of Earth, located above the troposphere and below the mesosphere. The stratosphere is an atmospheric layer composed of stratified temperature layers, with the warm layers of air high in the sky and the cool layers of air in the low sky, close to the planetary surface of the Earth. The increase of temperature with altitude is a result of the absorption of the Sun's ultraviolet (UV) radiation by the ozone layer. The temperature inversion is in contrast to the troposphere, and near the Earth's surface, where temperature decreases with altitude.

The tropopause is the atmospheric boundary that demarcates the troposphere from the stratosphere, which are the lowest two of the five layers of the atmosphere of Earth. The tropopause is a thermodynamic gradient-stratification layer that marks the end of the troposphere, and is approximately 17 kilometres (11 mi) above the equatorial regions, and approximately 9 kilometres (5.6 mi) above the polar regions.

Peroxyacetyl nitrate is a peroxyacyl nitrate. It is a secondary pollutant present in photochemical smog. It is thermally unstable and decomposes into peroxyethanoyl radicals and nitrogen dioxide gas. It is a lachrymatory substance, meaning that it irritates the lungs and eyes.

Ground-level ozone (O3), also known as surface-level ozone and tropospheric ozone, is a trace gas in the troposphere (the lowest level of the Earth's atmosphere), with an average concentration of 20–30 parts per billion by volume (ppbv), with close to 100 ppbv in polluted areas. Ozone is also an important constituent of the stratosphere, where the ozone layer (2 to 8 parts per million ozone) exists which is located between 10 and 50 kilometers above the Earth's surface. The troposphere extends from the ground up to a variable height of approximately 14 kilometers above sea level. Ozone is least concentrated in the ground layer (or planetary boundary layer) of the troposphere. Ground-level or tropospheric ozone is created by chemical reactions between NOx gases (oxides of nitrogen produced by combustion) and volatile organic compounds (VOCs). The combination of these chemicals in the presence of sunlight form ozone. Its concentration increases as height above sea level increases, with a maximum concentration at the tropopause. About 90% of total ozone in the atmosphere is in the stratosphere, and 10% is in the troposphere. Although tropospheric ozone is less concentrated than stratospheric ozone, it is of concern because of its health effects. Ozone in the troposphere is considered a greenhouse gas, and may contribute to global warming.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.

Paul Jozef Crutzen was a Dutch meteorologist and atmospheric chemist. He and Mario Molina and Frank Sherwood Rowland were awarded the Nobel Prize in Chemistry in 1995 for their work on atmospheric chemistry and specifically for his efforts in studying the formation and decomposition of atmospheric ozone. In addition to studying the ozone layer and climate change, he popularized the term Anthropocene to describe a proposed new epoch in the Quaternary period when human actions have a drastic effect on the Earth. He was also amongst the first few scientists to introduce the idea of a nuclear winter to describe the potential climatic effects stemming from large-scale atmospheric pollution including smoke from forest fires, industrial exhausts, and other sources like oil fires.
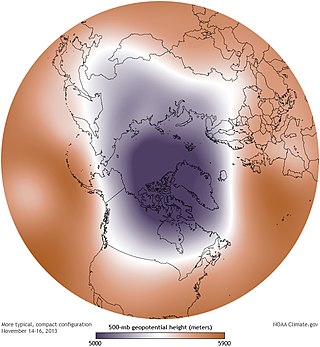
A circumpolar vortex, or simply polar vortex, is a large region of cold, rotating air; polar vortices encircle both of Earth's polar regions. Polar vortices also exist on other rotating, low-obliquity planetary bodies. The term polar vortex can be used to describe two distinct phenomena; the stratospheric polar vortex, and the tropospheric polar vortex. The stratospheric and tropospheric polar vortices both rotate in the direction of the Earth's spin, but they are distinct phenomena that have different sizes, structures, seasonal cycles, and impacts on weather.

The hydroperoxyl radical, also known as the hydrogen superoxide, is the protonated form of superoxide with the chemical formula HO2, also written HOO•. This species plays an important role in the atmosphere and as a reactive oxygen species in cell biology.

Vanadium bromoperoxidases are a kind of enzymes called haloperoxidases. Its primary function is to remove hydrogen peroxide which is produced during photosynthesis from in or around the cell. By producing hypobromous acid (HOBr) a secondary reaction with dissolved organic matter, what results is the bromination of organic compounds that are associated with the defense of the organism. These enzymes produce the bulk of natural organobromine compounds in the world.

Iodine oxides are chemical compounds of oxygen and iodine. Iodine has only two stable oxides which are isolatable in bulk, iodine tetroxide and iodine pentoxide, but a number of other oxides are formed in trace quantities or have been hypothesized to exist. The chemistry of these compounds is complicated with only a few having been well characterized. Many have been detected in the atmosphere and are believed to be particularly important in the marine boundary layer.
Equivalent effective stratospheric chlorine (EESC) provides an estimate of the total effective amount of halogens in the stratosphere. It is calculated from emission of chlorofluorocarbon and related halogenated compounds into the troposphere and their efficiency in contributing to stratospheric ozone depletion, and by making assumptions on transport times into the upper atmosphere (stratosphere). This parameter is used to quantify man-made ozone depletion and its changes with time. As a consequence of the Montreal Protocol and its amendments phasing out ozone-depleting substances (ODS), the EESC reached maximum in the late 1990s and is now slowly decreasing.

Costas Varotsos is a Greek physicist known from his contribution to the global climate-dynamics research and remote sensing.
Barbara J. Finlayson-Pitts is a Canadian-American atmospheric chemist. She is a professor in the chemistry department at the University of California, Irvine and is the Director of AirUCI Institute. Finlayson-Pitts and James N. Pitts, Jr. are the authors of Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications (1999). She has been a member of the National Academy of Sciences since 2006 and is the laureate for the 2017 Garvan–Olin Medal. In 2016 she co-chaired the National Academy of Science report "The Future of Atmospheric Chemistry Research"
Lucy Jane Carpenter is professor of physical chemistry at the University of York and director of the Cape Verde Atmospheric Observatory (CVAO).

Julia Yvonne Schmale is a German environmental scientist. She is a specialist in the micro-physical makeup of the atmosphere, in particular aerosols and their interaction with clouds. She is a professor at EPFL and the head of the Extreme Environments Research Laboratory (EERL). She is a participant in the Multidisciplinary drifting Observatory for the Study of Arctic Climate (MOSAiC) expeditions.

The bromine cycle is a biogeochemical cycle of bromine through the atmosphere, biosphere, and hydrosphere.
Jennifer Logan is an atmospheric scientist known for her research on how human activities influence the atmosphere, particularly with respect to biomass burning and the ozone hole.
John Maurice Campbell Plane,, , is a British atmospheric chemist, currently Professor of Atmospheric Chemistry at the University of Leeds. His research investigates planetary atmospheres using a range of theoretical and experimental techniques.















