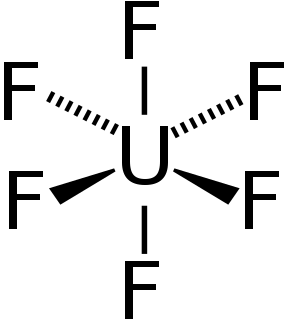
Uranium hexafluoride (UF
6), (sometimes called "hex") is an inorganic compound with the formula UF6. Uranium hexafluoride is a volatile white solid that reacts with water, releasing corrosive hydrofluoric acid. The compound reacts mildly with aluminium, forming a thin surface layer of AlF3 that resists any further reaction from the compound. UF6 is used in the process of enriching uranium, which produces fuel for nuclear reactors and nuclear weapons.
Tungsten(VI) fluoride, also known as tungsten hexafluoride, is an inorganic compound with the formula WF6. It is a toxic, corrosive, colorless gas, with a density of about 13 grams per litre (0.00047 lb/cu in) (roughly 11 times heavier than air. It is one of the densest known gases under standard conditions. WF6 ls commonly used by the semiconductor industry to form tungsten films, through the process of chemical vapor deposition. This layer is used in a low-resistivity metallic "interconnect". It is one of seventeen known binary hexafluorides.

Tungsten(VI) oxide, also known as tungsten trioxide is a chemical compound of oxygen and the transition metal tungsten, with formula WO3. The compound is also called tungstic anhydride, reflecting its relation to tungstic acid H2WO4. It is a light yellow crystalline solid.

Sodium tungstate is the inorganic compound with the formula Na2WO4. This white, water-soluble solid is the sodium salt of tungstic acid. It is useful as a source of tungsten for chemical synthesis. It is an intermediate in the conversion of tungsten ores to the metal.

Molybdenum(V) chloride is the inorganic compound with the empirical formula MoCl5. This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents.

Tungsten hexachloride is the chemical compound of tungsten and chlorine with the formula WCl6. This dark violet blue species exists as a volatile solid under standard conditions. It is an important starting reagent in the preparation of tungsten compounds. Other examples of charge-neutral hexachlorides are ReCl6 and MoCl6. The highly volatile WF6 is also known.

Tungsten(V) bromide is the inorganic compound with the empirical formula WBr5. The compound consists of bioctahedral structure, with two bridging bromide ligands, so its molecular formula is W2Br10.

Tungsten dichloride dioxide, or Tungstyl chloride is the chemical compound with the formula WO2Cl2. It is a yellow-colored solid. It is used as a precursor to other tungsten compounds. Like other tungsten halides, WO2Cl2 is sensitive to moisture, undergoing hydrolysis.
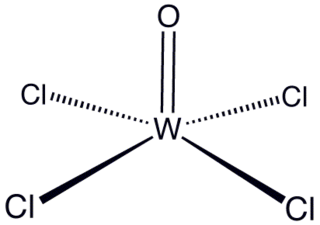
Tungsten(VI) oxytetrachloride is the inorganic compound with the formula WOCl4. This diamagnetic solid is used to prepare other complexes of tungsten. The yellow-green compound is soluble in nonpolar solvents but it reacts with alcohols and water and forms adducts with Lewis bases.
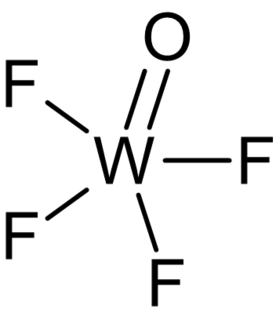
Tungsten(VI) oxytetrafluoride (WOF4) is an inorganic chemical compound.
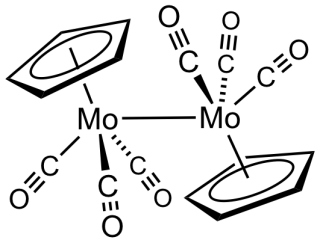
Cyclopentadienylmolybdenum tricarbonyl dimer is the chemical compound with the formula Cp2Mo2(CO)6, where Cp is C5H5. A dark red solid, it has been the subject of much research although it has no practical uses.

Hexamethyltungsten is the chemical compound W(CH3)6 also written WMe6. Classified as a transition metal alkyl complex, hexamethyltungsten is an air-sensitive, red, crystalline solid at room temperature; however, it is extremely volatile and sublimes at −30 °C. Owing to its six methyl groups it is extremely soluble in petroleum, aromatic hydrocarbons, ethers, carbon disulfide, and carbon tetrachloride.

Molybdenum hexafluoride, also molybdenum(VI) fluoride, is the inorganic compound with the formula MoF6. It is the highest fluoride of molybdenum. A colourless solid, it melts just below room temperature and boils in 34 °C. It is one of the seventeen known binary hexafluorides.
Vanadium(III) iodide is the inorganic compound with the formula VI3. This paramagnetic solid is generated by the reaction of vanadium powder with iodine at around 500 °C. The black hygroscopic crystals dissolve in water to give green solutions, characteristic of V(III) ions.

Niobium(IV) chloride, also known as niobium tetrachloride, is the chemical compound of formula NbCl4. This compound exists as dark violet crystals, is highly sensitive to air and moisture, and disproportiates into niobium(III) chloride and niobium(V) chloride when heated.

Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common.
Tungsten tetrafluoride is an inorganic compound with the formula WF4. This little studied solid has been invoked, together with tungsten pentafluoride, as an intermediate in the chemical vapor deposition of tungsten films using tungsten hexafluoride.
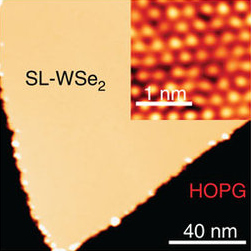
Tungsten diselenide is an inorganic compound with the formula WSe2. The compound adopts a hexagonal crystalline structure similar to molybdenum disulfide. Every tungsten atom is covalently bonded to six selenium ligands in a trigonal prismatic coordination sphere while each selenium is bonded to three tungsten atoms in a pyramidal geometry. The tungsten–selenium bond has a length of 0.2526 nm, and the distance between selenium atoms is 0.334 nm. It is a well studied example of a layered material. The layers stack together via van der Waals interactions. WSe2 is a very stable semiconductor in the group-VI transition metal dichalcogenides.

Tungsten(III) chloride is the inorganic compound with the formula W6Cl18. It is a cluster compound. It is a brown solid, obtainable by chlorination of tungsten(II) chloride. Featuring twelve doubly bridging chloride ligands, the cluster adopts a structure related to the corresponding chlorides of niobium and tantalum. In contrast, W6Cl12 features eight triply bridging chlorides.

Cyclopentadienyltungsten tricarbonyl dimer is the organotungsten compound with the formula Cp2W2(CO)6, where Cp is C5H5. A dark red crystalline solid, it is the subject of research, although it has no or few practical uses.
















