 | |
| Names | |
|---|---|
| Preferred IUPAC name 4-(2-Hydroxyethyl)phenol | |
| Other names p-Hydroxyphenethyl alcohol 2-(4-Hydroxyphenyl)ethanol 4-Hydroxyphenylethanol | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.210 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C8H10O2 | |
| Molar mass | 138.164 g/mol |
| Melting point | 91 to 92 °C (196 to 198 °F; 364 to 365 K) |
| Boiling point | 158 °C (316 °F; 431 K) at 4 Torr |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
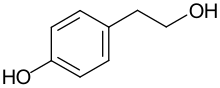
Tyrosol is an organic compound with the formula HOC6H4CH2CH2OH. Classified as a phenylethanoid, a derivative of phenethyl alcohol, it is found in a variety of natural sources. The compound is colorless solid. The principal source in the human diet is olive oil. [1] [2]