Related Research Articles

The endoplasmic reticulum (ER) is a part of a transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae, and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa.

The endomembrane system is composed of the different membranes (endomembranes) that are suspended in the cytoplasm within a eukaryotic cell. These membranes divide the cell into functional and structural compartments, or organelles. In eukaryotes the organelles of the endomembrane system include: the nuclear membrane, the endoplasmic reticulum, the Golgi apparatus, lysosomes, vesicles, endosomes, and plasma (cell) membrane among others. The system is defined more accurately as the set of membranes that forms a single functional and developmental unit, either being connected directly, or exchanging material through vesicle transport. Importantly, the endomembrane system does not include the membranes of plastids or mitochondria, but might have evolved partially from the actions of the latter.

The Golgi apparatus, also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus.
Protein targeting or protein sorting is the biological mechanism by which proteins are transported to their appropriate destinations within or outside the cell. Proteins can be targeted to the inner space of an organelle, different intracellular membranes, the plasma membrane, or to the exterior of the cell via secretion. Information contained in the protein itself directs this delivery process. Correct sorting is crucial for the cell; errors or dysfunction in sorting have been linked to multiple diseases.

In cell biology, a vesicle is a structure within or outside a cell, consisting of liquid or cytoplasm enclosed by a lipid bilayer. Vesicles form naturally during the processes of secretion (exocytosis), uptake (endocytosis), and the transport of materials within the plasma membrane. Alternatively, they may be prepared artificially, in which case they are called liposomes. If there is only one phospholipid bilayer, the vesicles are called unilamellar liposomes; otherwise they are called multilamellar liposomes. The membrane enclosing the vesicle is also a lamellar phase, similar to that of the plasma membrane, and intracellular vesicles can fuse with the plasma membrane to release their contents outside the cell. Vesicles can also fuse with other organelles within the cell. A vesicle released from the cell is known as an extracellular vesicle.

Exocytosis is a form of active transport and bulk transport in which a cell transports molecules out of the cell. As an active transport mechanism, exocytosis requires the use of energy to transport material. Exocytosis and its counterpart, endocytosis, are used by all cells because most chemical substances important to them are large polar molecules that cannot pass through the hydrophobic portion of the cell membrane by passive means. Exocytosis is the process by which a large amount of molecules are released; thus it is a form of bulk transport. Exocytosis occurs via secretory portals at the cell plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structures at the cell plasma membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell.
A cisterna is a flattened membrane vesicle found in the endoplasmic reticulum and Golgi apparatus. Cisternae are an integral part of the packaging and modification processes of proteins occurring in the Golgi.
A signal peptide is a short peptide present at the N-terminus of most newly synthesized proteins that are destined toward the secretory pathway. These proteins include those that reside either inside certain organelles, secreted from the cell, or inserted into most cellular membranes. Although most type I membrane-bound proteins have signal peptides, most type II and multi-spanning membrane-bound proteins are targeted to the secretory pathway by their first transmembrane domain, which biochemically resembles a signal sequence except that it is not cleaved. They are a kind of target peptide.
The Coat Protein Complex II, or COPII, is a group of proteins that facilitate the formation of vesicles to transport proteins from the endoplasmic reticulum to the Golgi apparatus or endoplasmic-reticulum–Golgi intermediate compartment. This process is termed anterograde transport, in contrast to the retrograde transport associated with the COPI complex. COPII is assembled in two parts: first an inner layer of Sar1, Sec23, and Sec24 forms; then the inner coat is surrounded by an outer lattice of Sec13 and Sec31.

COPI is a coatomer, a protein complex that coats vesicles transporting proteins from the cis end of the Golgi complex back to the rough endoplasmic reticulum (ER), where they were originally synthesized, and between Golgi compartments. This type of transport is retrograde transport, in contrast to the anterograde transport associated with the COPII protein. The name "COPI" refers to the specific coat protein complex that initiates the budding process on the cis-Golgi membrane. The coat consists of large protein subcomplexes that are made of seven different protein subunits, namely α, β, β', γ, δ, ε and ζ.
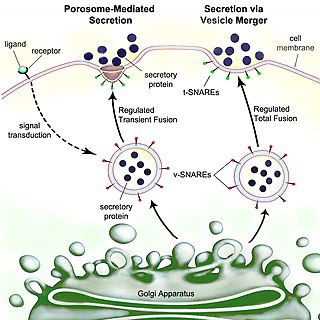
Secretion is the movement of material from one point to another, such as a secreted chemical substance from a cell or gland. In contrast, excretion is the removal of certain substances or waste products from a cell or organism. The classical mechanism of cell secretion is via secretory portals at the plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structures embedded in the cell membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell.

Dense granules are specialized secretory organelles. Dense granules are found only in platelets and are smaller than alpha granules. The origin of these dense granules is still unknown, however, it is thought that may come from the mechanism involving the endocytotic pathway. Dense granules are a sub group of lysosome-related organelles (LRO). There are about three to eight of these in a normal human platelet.
Endoplasm generally refers to the inner, dense part of a cell's cytoplasm. This is opposed to the ectoplasm which is the outer (non-granulated) layer of the cytoplasm, which is typically watery and immediately adjacent to the plasma membrane. The nucleus is separated from the endoplasm by the nuclear envelope. The different makeups/viscosities of the endoplasm and ectoplasm contribute to the amoeba's locomotion through the formation of a pseudopod. However, other types of cells have cytoplasm divided into endo- and ectoplasm. The endoplasm, along with its granules, contains water, nucleic acids, amino acids, carbohydrates, inorganic ions, lipids, enzymes, and other molecular compounds. It is the site of most cellular processes as it houses the organelles that make up the endomembrane system, as well as those that stand alone. The endoplasm is necessary for most metabolic activities, including cell division.
A secretory protein is any protein, whether it be endocrine or exocrine, which is secreted by a cell. Secretory proteins include many hormones, enzymes, toxins, and antimicrobial peptides. Secretory proteins are synthesized in the endoplasmic reticulum.
Cell physiology is the biological study of the activities that take place in a cell to keep it alive. The term physiology refers to normal functions in a living organism. Animal cells, plant cells and microorganism cells show similarities in their functions even though they vary in structure.
The coatomer is a protein complex that coats membrane-bound transport vesicles. Two types of coatomers are known:
Reticulons are a group of evolutionary conservative proteins residing predominantly in endoplasmic reticulum, primarily playing a role in promoting membrane curvature. In addition, reticulons may play a role in nuclear pore complex formation, vesicle formation, and other processes yet to be defined. They have also been linked to oligodendrocyte roles in inhibition of neurite outgrowth. Some studies link RTNs with Alzheimer's disease and amyotrophic lateral sclerosis.
KDEL is a target peptide sequence in mammals and plants located on the C-terminal end of the amino acid structure of a protein. The KDEL sequence prevents a protein from being secreted from the endoplasmic reticulum (ER) and facilitates its return if it is accidentally exported.
A target peptide is a short peptide chain that directs the transport of a protein to a specific region in the cell, including the nucleus, mitochondria, endoplasmic reticulum (ER), chloroplast, apoplast, peroxisome and plasma membrane. Some target peptides are cleaved from the protein by signal peptidases after the proteins are transported.
Membrane vesicle trafficking in eukaryotic animal cells involves movement of biochemical signal molecules from synthesis-and-packaging locations in the Golgi body to specific release locations on the inside of the plasma membrane of the secretory cell. It takes place in the form of Golgi membrane-bound micro-sized vesicles, termed membrane vesicles (MVs).
References
- 1 2 Backhaus R, Zehe C, Wegehingel S, Kehlenbach A, Schwappach B, Nickel W (April 2004). "Unconventional protein secretion: membrane translocation of FGF-2 does not require protein unfolding". Journal of Cell Science. 117 (Pt 9): 1727–1736. doi:10.1242/jcs.01027. PMID 15075234.
- 1 2 3 4 Nickel W, Rabouille C (February 2009). "Mechanisms of regulated unconventional protein secretion". Nature Reviews. Molecular Cell Biology. 10 (2): 148–155. doi:10.1038/nrm2617. PMID 19122676. S2CID 9824754.
- ↑ Nickel W (October 2010). "Pathways of unconventional protein secretion". Current Opinion in Biotechnology. 21 (5): 621–626. doi:10.1016/j.copbio.2010.06.004. PMID 20637599.