Related Research Articles

The pharmaceutical industry discovers, develops, produces, and markets drugs or pharmaceutical drugs for use as medications to be administered to patients, with the aim to cure them, vaccinate them, or alleviate symptoms. Pharmaceutical companies may deal in generic or brand medications and medical devices. They are subject to a variety of laws and regulations that govern the patenting, testing, safety, efficacy using drug testing and marketing of drugs. The global pharmaceuticals market produced treatments worth $1,228.45 billion in 2020 and showed a compound annual growth rate (CAGR) of 1.8%.
Growth hormone therapy refers to the use of growth hormone (GH) as a prescription medication—it is one form of hormone therapy. Growth hormone is a peptide hormone secreted by the pituitary gland that stimulates growth and cell reproduction. In the past, growth hormone was extracted from human pituitary glands. Growth hormone is now produced by recombinant DNA technology and is prescribed for a variety of reasons. GH therapy has been a focus of social and ethical controversies for 50 years.
Drotrecogin alfa (activated) (Xigris, marketed by Eli Lilly and Company) is a recombinant form of human activated protein C that has anti-thrombotic, anti-inflammatory, and profibrinolytic properties. Drotrecogin alpha (activated) belongs to the class of serine proteases. Drotrecogin alfa has not been found to improve outcomes in people with severe sepsis. The manufacturer's aggressive strategies in marketing its use in severe sepsis have been criticized. On October 25, 2011, Eli Lilly & Co. withdrew Xigris from the market after a major study showed no efficacy for the treatment of sepsis.

Chenodeoxycholic acid is a bile acid. Salts of this carboxylic acid are called chenodeoxycholates. Chenodeoxycholic acid is one of the main bile acids. It was first isolated from the bile of the domestic goose, which gives it the "cheno" portion of its name.
Hydralazine/isosorbide dinitrate, sold under the brand name Bidil, is a fixed-dose combination medication used to treat self-identified Black people with congestive heart failure. It is a combination of hydralazine hydrochloride and isosorbide dinitrate.

Paul M. Ridker is a cardiovascular epidemiologist and biomedical researcher. He is currently the Eugene Braunwald Professor of Medicine at Harvard University and Brigham and Women's Hospital, where he directs the Center for Cardiovascular Disease Prevention. Ridker also holds an appointment as Professor in the Department of Epidemiology at the Harvard T.H. Chan School of Public Health.
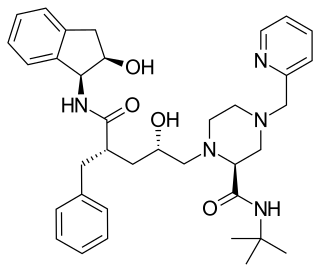
Indinavir is a protease inhibitor used as a component of highly active antiretroviral therapy to treat HIV/AIDS. It is soluble white powder administered orally in combination with other antiviral drugs. The drug prevents protease from functioning normally. Consequently, HIV viruses cannot reproduce, causing a decrease in the viral load. Commercially sold indinavir is indinavir anhydrous, which is indinavir with an additional amine in the hydroxyethylene backbone. This enhances its solubility and oral bioavailability, making it easier for users to intake. It was synthetically produced for the purpose of inhibiting the protease in the HIV virus.

Juice Plus is a branded line of dietary supplements. It is produced by Natural Alternatives International of San Marcos, California, for National Safety Associates. Introduced in 1993, the supplements are distributed by NSA via multi-level marketing. Juice Plus supplements contain fruit and vegetable juice extracts with added vitamins and nutrients.

Marcia Angell is an American physician, author, and the first woman to serve as editor-in-chief of the New England Journal of Medicine. She is currently a Senior Lecturer in the Department of Global Health and Social Medicine at Harvard Medical School in Boston, Massachusetts.
David Lawrence Sackett was an American-Canadian physician and a pioneer in evidence-based medicine. He is known as one of the fathers of Evidence-Based Medicine. He founded the first department of clinical epidemiology in Canada at McMaster University, and the Oxford Centre for Evidence-Based Medicine. He is well known for his textbooks Clinical Epidemiology and Evidence-Based Medicine.
The history of invasive and interventional cardiology is complex, with multiple groups working independently on similar technologies. Invasive and interventional cardiology is currently closely associated with cardiologists, though the development and most of its early research and procedures were performed by diagnostic and interventional radiologists.
John A. Wise, PhD (1939–2011), was an American scientist. He retired in 2010 as the Chief Science Officer for Natural Alternatives International (NAI), a nutritional products manufacturer based in San Marcos, California USA. Earlier in his career, he was the Vice-president of Research and Development for United Sciences of America, Inc. (USAI) until 1986. USAI was a multi-level marketing company in Dallas, Texas which went bankrupt after controversial marketing practices were revealed.
Jerris Leonard was an American lawyer and Republican politician. He served 8 years in the Wisconsin State Senate (1961–1969) and four years in the State Assembly (1957–1961), representing northern Milwaukee County.

Alogliptin, sold under the brand names Nesina and Vipidia,) is an oral anti-diabetic drug in the DPP-4 inhibitor (gliptin) class. Alogliptin does not decrease the risk of heart attack and stroke. Like other members of the gliptin class, it causes little or no weight gain, exhibits relatively little risk of hypoglycemia, and has relatively modest glucose-lowering activity. Alogliptin and other gliptins are commonly used in combination with metformin in people whose diabetes cannot adequately be controlled with metformin alone.

MonaVie is a defunct, American multi-level marketing company that manufactured and distributed products made from blended fruit juice concentrates, powders, and purées. The company was the subject of several controversies. Health claims for its products had not been scientifically confirmed or approved by regulatory authorities, and its chairman had been previously involved in false health claims concerning another beverage company. According to Forbes, MonaVie's business plan resembled a pyramid scheme. In 2015, the company defaulted on a US$182 million loan and went into foreclosure. Florida-based Jeunesse Global took over MonaVie’s assets when it purchased the note for $15 million.

Telaprevir (VX-950), marketed under the brand names Incivek and Incivo, is a pharmaceutical drug for the treatment of hepatitis C co-developed by Vertex Pharmaceuticals and Johnson & Johnson. It is a member of a class of antiviral drugs known as protease inhibitors. Specifically, telaprevir inhibits the hepatitis C viral enzyme NS3/4A serine protease. Telaprevir is only indicated for use against hepatitis C genotype 1 viral infections and has not been proven to be safe or effective when used for other genotypes of the virus. The standard therapy of pegylated interferon and ribavirin is less effective than telaprevir in those with genotype 1.
Michael Stuart Gottlieb is an American physician and immunologist known for his 1981 identification of acquired immune deficiency syndrome (AIDS) as a new disease, and for his HIV/AIDS research, HIV/AIDS activism, and philanthropic efforts associated with HIV/AIDS treatment.
Alirocumab, sold under the brand name Praluent, is a medication used as a second-line treatment for high cholesterol for adults whose cholesterol is not controlled by diet and statin treatment. It is a human monoclonal antibody that belongs to a novel class of anti-cholesterol drugs, known as PCSK9 inhibitors, and it was the first such agent to receive FDA approval. The FDA approval was contingent on the completion of further clinical trials to better determine efficacy and safety.
Cytochrome b5 deficiency is a rare condition and form of isolated 17,20-lyase deficiency caused by deficiency in cytochrome b5, a small hemoprotein that acts as an allosteric factor to facilitate the interaction of CYP17A1 (17α-hydroxylase/17,20-lyase) with P450 oxidoreductase (POR), thereby allowing for the 17,20-lyase activity of CYP17A1. The condition affects both adrenal and gonadal androgen biosynthesis and results in male pseudohermaphroditism. The principal biological role of cytochrome b5 is reduction of methemoglobin, so cytochrome b5 deficiency can also result in elevated methemoglobin levels and/or methemoglobinemia, similarly to deficiency of cytochrome b5 reductase.
Jean Holowach Thurston was an American pediatric neurologist known for her research on epilepsy. She was a professor of pediatrics at Washington University School of Medicine from 1949 until her retirement in 1987. In 2004, she won the first Roger and Mary Brumback Lifetime Achievement Award from the Child Neurology Society.
References
- 1 2 3 4 "USA: The strange rise and fall of one MLM". Money (June 1). 1987.
- 1 2 Farley, Dixie (1987). "The eyes of Texas were upon them - and FDA - United Sciences of America Inc". FDA Consumer (October).
- ↑ Stare, F.J.; . (1986). "Marketing a nutritional "revolutionary breakthrough". Trading on names". N Engl J Med. 315 (15): 971–3. doi:10.1056/NEJM198610093151518. PMID 3762604.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ↑ Young, E.A.; Schenker, S.; Weser, E. (1987). "United Sciences of America, Incorporated: an "optimal" diet?". Ann Intern Med. 107 (1): 101–3. doi:10.7326/0003-4819-107-1-101. PMID 3592422.
- ↑ Renner, J.H. (1986). "Science or scam?". N Engl J Med. 315 (15): 971. doi:10.1056/NEJM198610093151517. PMID 3762603.
- ↑ Holden, C. (1986). "Scientists get flak over marketing plan". Science. 234 (4780): 1063–4. doi:10.1126/science.3775374. PMID 3775374.
- ↑ Blodgett, N (1987). "State tackles health care frauds". ABA Journal. Oct 1, 1987: 32.