Related Research Articles
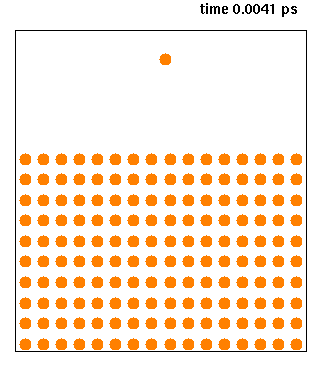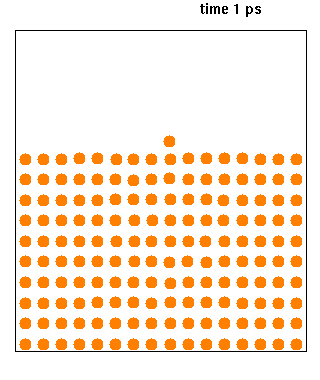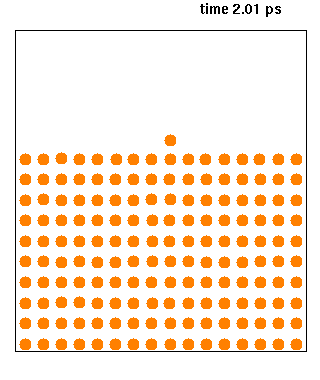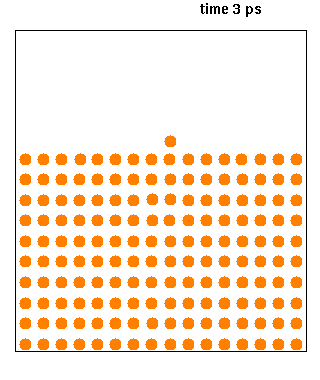
Molecular dynamics (MD) is a computer simulation method for analyzing the physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the dynamic "evolution" of the system. In the most common version, the trajectories of atoms and molecules are determined by numerically solving Newton's equations of motion for a system of interacting particles, where forces between the particles and their potential energies are often calculated using interatomic potentials or molecular mechanical force fields. The method is applied mostly in chemical physics, materials science, and biophysics.
Chemistry at Harvard Macromolecular Mechanics (CHARMM) is the name of a widely used set of force fields for molecular dynamics, and the name for the molecular dynamics simulation and analysis computer software package associated with them. The CHARMM Development Project involves a worldwide network of developers working with Martin Karplus and his group at Harvard to develop and maintain the CHARMM program. Licenses for this software are available, for a fee, to people and groups working in academia.
Systems Science, also referred to as systems research, or, simply, systems, is a transdisciplinary field that is concerned with understanding simple and complex systems in nature and society, for which leads to the advancements of formal, natural, social, and applied attributions throughout engineering, technology and science, itself.
In the context of chemistry, molecular physics and physical chemistry and molecular modelling, a force field is a computational model that is used to describe the forces between atoms within molecules or between molecules as well as in crystals. Force fields are a variety of interatomic potentials. More precisely, the force field refers to the functional form and parameter sets used to calculate the potential energy of a system of the atomistic level. Force fields are usually used in molecular dynamics or Monte Carlo simulations. The parameters for a chosen energy function may be derived from classical laboratory experiment data, calculations in quantum mechanics, or both. Force fields utilize the same concept as force fields in classical physics, with the main difference that the force field parameters in chemistry describe the energy landscape on the atomistic level. From a force field, the acting forces on every particle are derived as a gradient of the potential energy with respect to the particle coordinates.

Michael Levitt, is a South African-born biophysicist and a professor of structural biology at Stanford University, a position he has held since 1987. Levitt received the 2013 Nobel Prize in Chemistry, together with Martin Karplus and Arieh Warshel, for "the development of multiscale models for complex chemical systems". In 2018, Levitt was a founding co-editor of the Annual Review of Biomedical Data Science.

Martin Karplus is an Austrian and American theoretical chemist. He is the Director of the Biophysical Chemistry Laboratory, a joint laboratory between the French National Center for Scientific Research and the University of Strasbourg, France. He is also the Theodore William Richards Professor of Chemistry, emeritus at Harvard University. Karplus received the 2013 Nobel Prize in Chemistry, together with Michael Levitt and Arieh Warshel, for "the development of multiscale models for complex chemical systems".

William (Bill) DeGrado is a professor at the University of California, San Francisco, where he is the Toby Herfindal Presidential Professor of Entrepreneurship and Innovation in the Department of Pharmaceutical Chemistry. As an early pioneer of protein design, he coined the term de novo protein design. He is also active in discovery of small molecule drugs for a variety of human diseases. He is a member of the U.S. National Academy of Sciences (1999), American Academy of Arts & Sciences (1997) and National Academy of Inventors. He also is a scientific cofounder of Pliant therapeutics.

Harry Barkus Gray is the Arnold O. Beckman Professor of Chemistry at California Institute of Technology.

Jennifer Lippincott-Schwartz is a Senior Group Leader at Howard Hughes Medical Institute's Janelia Research Campus and a founding member of the Neuronal Cell Biology Program at Janelia. Previously, she was the Chief of the Section on Organelle Biology in the Cell Biology and Metabolism Program, in the Division of Intramural Research in the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health from 1993 to 2016. Lippincott-Schwartz received her PhD from Johns Hopkins University, and performed post-doctoral training with Richard Klausner at the NICHD, NIH in Bethesda, Maryland.
Bernhard Örn Pálsson is the Galletti Professor of Bioengineering and an adjunct professor of Medicine at the University of California, San Diego.
Linda Carol Hsieh-Wilson is an American chemist and the Milton and Rosalind Chang Professor of Chemistry at the California Institute of Technology. She is known for her work in chemical neurobiology on understanding the structure and function of carbohydrates in the nervous system. Her studies have revealed critical roles for carbohydrates and protein glycosylation in fundamental processes ranging from cellular metabolism to memory storage. She is a member of the American Academy of Arts and Sciences and was elected to the National Academy of Sciences in 2022.

Martin Gruebele is a German-born American physical chemist and biophysicist who is currently James R. Eiszner Professor of Chemistry, Professor of Physics, Professor of Biophysics and Computational Biology at the University of Illinois Urbana-Champaign, where he is the principal investigator of the Gruebele Group.
Naomi Shauna Ginsberg is a Canadian electrical engineer, physicist, and scientist. She is currently an associate professor of chemistry at the University of California, Berkeley.
Stephen H. White is an American Biophysicist, academic, and author. He is a Professor Emeritus of Physiology and Biophysics at the University of California, Irvine.

Sarah Amalia Teichmann is a German scientist who is head of cellular genetics at the Wellcome Sanger Institute and a visiting research group leader at the European Bioinformatics Institute (EMBL-EBI). She serves as director of research in the Cavendish Laboratory, at the University of Cambridge and a senior research fellow at Churchill College, Cambridge.

Rong Li is the Director of Mechanobiology Institute, a Singapore Research Center of Excellence, at the National University of Singapore. She is a Distinguished Professor at the National University of Singapore's Department of Biological Sciences and Bloomberg Distinguished Professor of Cell Biology and Chemical & Biomolecular Engineering at the Johns Hopkins School of Medicine and Whiting School of Engineering. She previously served as Director of Center for Cell Dynamics in the Johns Hopkins School of Medicine’s Institute for Basic Biomedical Sciences. She is a leader in understanding cellular asymmetry, division and evolution, and specifically, in how eukaryotic cells establish their distinct morphology and organization in order to carry out their specialized functions.

Biman Bagchi is an Indian scientist currently serving as a SERB-DST National Science Chair Professor and Honorary Professor at the Solid State and Structural Chemistry Unit of the Indian Institute of Science. He is a theoretical physical chemist and biophysicist known for his research in the area of statistical mechanics; particularly in the study of phase transition and nucleation, solvation dynamics, mode-coupling theory of electrolyte transport, dynamics of biological macromolecules, protein folding, enzyme kinetics, supercooled liquids and protein hydration layer. He is an elected fellow of the Indian National Science Academy, the Indian Academy of Sciences, The World Academy of Sciences and an International honorary member of the American Academy of Arts and Sciences. Along with several scientific articles, he has authored three books, (i) Molecular Relaxation in Liquids, (ii) Water in Biological and Chemical Processes: From Structure and Dynamics to Function, and (iii) Statistical Mechanics for Chemistry and Materials Science.
Teresa Lyn Head-Gordon is an American chemist and the Chancellor's Professor of Chemistry, Bioengineering, and Chemical and Biomolecular Engineering at the University of California, Berkeley. She is also a faculty scientist in the Chemical Sciences Division at the Lawrence Berkeley National Laboratory and a fellow of both the American Institute for Medical and Biological Engineering and the American Chemical Society (ACS).

In biochemistry, a backbone-dependent rotamer library provides the frequencies, mean dihedral angles, and standard deviations of the discrete conformations of the amino acid side chains in proteins as a function of the backbone dihedral angles φ and ψ of the Ramachandran map. By contrast, backbone-independent rotamer libraries express the frequencies and mean dihedral angles for all side chains in proteins, regardless of the backbone conformation of each residue type. Backbone-dependent rotamer libraries have been shown to have significant advantages over backbone-independent rotamer libraries, principally when used as an energy term, by speeding up search times of side-chain packing algorithms used in protein structure prediction and protein design.

Yu-Shan Lin is a computational chemist. She is an associate professor of chemistry at Tufts University in the United States. Her research lab uses computational chemistry to understand and design biomolecules, with topics focusing on cyclic peptides, protein folding, and collagen.
References
- ↑ Daggett, Valerie. "Daggett Research Group" . Retrieved 10 February 2015.
- ↑ "Team | AltPep". www.altpep.com. Retrieved 2021-11-17.
- ↑ Doerr, Alison (2010). "A database of dynamics". Nature Methods. 7 (6): 426. doi: 10.1038/nmeth0610-426 . PMID 20524218.
- ↑ Van Der Kamp, M. W.; Schaeffer, R. D.; Jonsson, A. L.; Scouras, A. D.; Simms, A. M.; Toofanny, R. D.; Benson, N. C.; Anderson, P. C.; Merkley, E. D.; Rysavy, S.; Bromley, D.; Beck, D. A. C.; Daggett, V. (2010). "Dynameomics: A Comprehensive Database of Protein Dynamics". Structure. 18 (4): 423–435. doi:10.1016/j.str.2010.01.012. PMC 2892689 . PMID 20399180.
- ↑ DOE press release on the awarding of INCITE grants. 22 Dec 2004. Access date 17 Jan 2007.
- ↑ Microsoft Corporation. "GrayWulf Takes Byte Out of Data Overload". Microsoft Research. Retrieved 10 February 2015.
- ↑ "2 0 1 9 H I S T O R I C A L I N F O R M A T I O N" (PDF). Retrieved November 17, 2021.
- ↑ Wright, Laura Elizabeth (26 January 2015). "Suzie Pun and Valerie Daggett elected AIMBE Fellows". University of Washington Department of Engineering. Retrieved 10 February 2015.