Related Research Articles

The putamen is a round structure located at the base of the forebrain (telencephalon). The putamen and caudate nucleus together form the dorsal striatum. It is also one of the structures that compose the basal nuclei. Through various pathways, the putamen is connected to the substantia nigra, the globus pallidus, the claustrum, and the thalamus, in addition to many regions of the cerebral cortex. A primary function of the putamen is to regulate movements at various stages and influence various types of learning. It employs GABA, acetylcholine, and enkephalin to perform its functions. The putamen also plays a role in degenerative neurological disorders, such as Parkinson's disease.

The striatum, or corpus striatum, is a nucleus in the subcortical basal ganglia of the forebrain. The striatum is a critical component of the motor and reward systems; receives glutamatergic and dopaminergic inputs from different sources; and serves as the primary input to the rest of the basal ganglia.

The basal ganglia (BG), or basal nuclei, are a group of subcortical nuclei, of varied origin, in the brains of vertebrates. In humans, and some primates, there are some differences, mainly in the division of the globus pallidus into an external and internal region, and in the division of the striatum. The basal ganglia are situated at the base of the forebrain and top of the midbrain. Basal ganglia are strongly interconnected with the cerebral cortex, thalamus, and brainstem, as well as several other brain areas. The basal ganglia are associated with a variety of functions, including control of voluntary motor movements, procedural learning, habit learning, conditional learning, eye movements, cognition, and emotion.
The mesolimbic pathway, sometimes referred to as the reward pathway, is a dopaminergic pathway in the brain. The pathway connects the ventral tegmental area in the midbrain to the ventral striatum of the basal ganglia in the forebrain. The ventral striatum includes the nucleus accumbens and the olfactory tubercle.

The globus pallidus (GP), also known as paleostriatum or dorsal pallidum, is a subcortical structure of the brain. It consists of two adjacent segments, one external, known in rodents simply as the globus pallidus, and one internal, known in rodents as the entopeduncular nucleus. It is part of the telencephalon, but retains close functional ties with the subthalamus in the diencephalon – both of which are part of the extrapyramidal motor system. The globus pallidus is a major component of the basal ganglia, with principal inputs from the striatum, and principal direct outputs to the thalamus and the substantia nigra. The latter is made up of similar neuronal elements, has similar afferents from the striatum, similar projections to the thalamus, and has a similar synaptology. Neither receives direct cortical afferents, and both receive substantial additional inputs from the intralaminar thalamus.

The nucleus accumbens is a region in the basal forebrain rostral to the preoptic area of the hypothalamus. The nucleus accumbens and the olfactory tubercle collectively form the ventral striatum. The ventral striatum and dorsal striatum collectively form the striatum, which is the main component of the basal ganglia. The dopaminergic neurons of the mesolimbic pathway project onto the GABAergic medium spiny neurons of the nucleus accumbens and olfactory tubercle. Each cerebral hemisphere has its own nucleus accumbens, which can be divided into two structures: the nucleus accumbens core and the nucleus accumbens shell. These substructures have different morphology and functions.
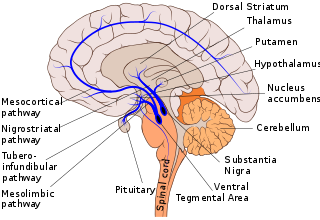
Dopaminergic pathways in the human brain are involved in both physiological and behavioral processes including movement, cognition, executive functions, reward, motivation, and neuroendocrine control. Each pathway is a set of projection neurons, consisting of individual dopaminergic neurons.

The nigrostriatal pathway is a bilateral dopaminergic pathway in the brain that connects the substantia nigra pars compacta (SNc) in the midbrain with the dorsal striatum in the forebrain. It is one of the four major dopamine pathways in the brain, and is critical in the production of movement as part of a system called the basal ganglia motor loop. Dopaminergic neurons of this pathway release dopamine from axon terminals that synapse onto GABAergic medium spiny neurons (MSNs), also known as spiny projection neurons (SPNs), located in the striatum.

The ventral tegmental area (VTA), also known as the ventral tegmental area of Tsai, or simply ventral tegmentum, is a group of neurons located close to the midline on the floor of the midbrain. The VTA is the origin of the dopaminergic cell bodies of the mesocorticolimbic dopamine system and other dopamine pathways; it is widely implicated in the drug and natural reward circuitry of the brain. The VTA plays an important role in a number of processes, including reward cognition and orgasm, among others, as well as several psychiatric disorders. Neurons in the VTA project to numerous areas of the brain, ranging from the prefrontal cortex to the caudal brainstem and several regions in between.

In neuroanatomy, habenula originally denoted the stalk of the pineal gland, but gradually came to refer to a neighboring group of nerve cells with which the pineal gland was believed to be associated, the habenular nucleus. The habenular nucleus is a set of well-conserved structures in all vertebrate animals.

The olfactory tubercle (OT), also known as the tuberculum olfactorium, is a multi-sensory processing center that is contained within the olfactory cortex and ventral striatum and plays a role in reward cognition. The OT has also been shown to play a role in locomotor and attentional behaviors, particularly in relation to social and sensory responsiveness, and it may be necessary for behavioral flexibility. The OT is interconnected with numerous brain regions, especially the sensory, arousal, and reward centers, thus making it a potentially critical interface between processing of sensory information and the subsequent behavioral responses.
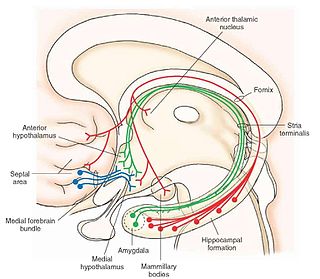
The medial forebrain bundle (MFB), is a neural pathway containing fibers from the basal olfactory regions, the periamygdaloid region and the septal nuclei, as well as fibers from brainstem regions, including the ventral tegmental area and nigrostriatal pathway.

The basal ganglia form a major brain system in all species of vertebrates, but in primates there are special features that justify a separate consideration. As in other vertebrates, the primate basal ganglia can be divided into striatal, pallidal, nigral, and subthalamic components. In primates, however, there are two pallidal subdivisions called the external globus pallidus (GPe) and internal globus pallidus (GPi). Also in primates, the dorsal striatum is divided by a large tract called the internal capsule into two masses named the caudate nucleus and the putamen—in most other species no such division exists, and only the striatum as a whole is recognized. Beyond this, there is a complex circuitry of connections between the striatum and cortex that is specific to primates. This complexity reflects the difference in functioning of different cortical areas in the primate brain.

Medium spiny neurons (MSNs), also known as spiny projection neurons (SPNs), are a special type of GABAergic inhibitory cell representing 95% of neurons within the human striatum, a basal ganglia structure. Medium spiny neurons have two primary phenotypes : D1-type MSNs of the direct pathway and D2-type MSNs of the indirect pathway. Most striatal MSNs contain only D1-type or D2-type dopamine receptors, but a subpopulation of MSNs exhibit both phenotypes.

The reward system is a group of neural structures responsible for incentive salience, associative learning, and positively-valenced emotions, particularly ones involving pleasure as a core component. Reward is the attractive and motivational property of a stimulus that induces appetitive behavior, also known as approach behavior, and consummatory behavior. A rewarding stimulus has been described as "any stimulus, object, event, activity, or situation that has the potential to make us approach and consume it is by definition a reward". In operant conditioning, rewarding stimuli function as positive reinforcers; however, the converse statement also holds true: positive reinforcers are rewarding.

The external globus pallidus combines with the internal globus pallidus (GPi) to form the globus pallidus, an anatomical subset of the basal ganglia. Globus pallidus means "pale globe" in Latin, indicating its appearance. The external globus pallidus is the segment of the globus pallidus that is relatively further (lateral) from the midline of the brain.

The internal globus pallidus and the external globus pallidus (GPe) make up the globus pallidus. The GPi is one of the output nuclei of the basal ganglia. The GABAergic neurons of the GPi send their axons to the ventral anterior nucleus (VA) and the ventral lateral nucleus (VL) in the dorsal thalamus, to the centromedian complex, and to the pedunculopontine complex.

Blocq's disease was first considered by Paul Blocq (1860–1896), who described this phenomenon as the loss of memory of specialized movements causing the inability to maintain an upright posture, despite normal function of the legs in the bed. The patient is able to stand up, but as soon as the feet are on the ground, the patient cannot hold himself upright nor walk; however when lying down, the subject conserved the integrity of muscular force and the precision of movements of the lower limbs. The motivation of this study came when a fellow student Georges Marinesco (1864) and Paul published a case of parkinsonian tremor (1893) due to a tumor located in the substantia nigra.
The rostromedial tegmental nucleus (RMTg), also known as the tail of the ventral tegmental area (tVTA), is a GABAergic nucleus which functions as a "master brake" for the midbrain dopamine system. It is poorly differentiated from the rest of the ventral tegmental area (VTA) and possesses robust functional and structural links to the dopamine pathways. Notably, both acute and chronic exposure to psychostimulants have been shown to induce FosB and ΔFosB expression in the RMTg; no other drug type has been shown to induce these proteins in the RMTg.
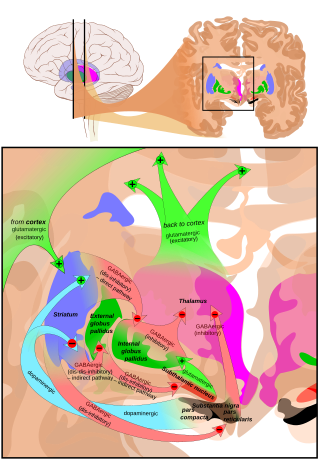
The cortico-basal ganglia-thalamo-cortical loop is a system of neural circuits in the brain. The loop involves connections between the cortex, the basal ganglia, the thalamus, and back to the cortex. It is of particular relevance to hyperkinetic and hypokinetic movement disorders, such as Parkinson's disease and Huntington's disease, as well as to mental disorders of control, such as attention deficit hyperactivity disorder (ADHD), obsessive–compulsive disorder (OCD), and Tourette syndrome.
References
- 1 2 Berridge KC, Kringelbach ML (May 2015). "Pleasure systems in the brain". Neuron. 86 (3): 646–664. doi:10.1016/j.neuron.2015.02.018. PMC 4425246 . PMID 25950633.
- ↑ Zahm, Daniel S. (2000-01-01). "An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens". Neuroscience & Biobehavioral Reviews. 24 (1): 85–105. doi:10.1016/S0149-7634(99)00065-2. ISSN 0149-7634.
- ↑ Smith Y, Keival JZ (2000). "Anatomy of the Dopamine system in the Basal Ganglia". Trends in Neurosciences. 23 (10): 28–33. doi:10.1016/S1471-1931(00)00023-9. PMID 11052217.
- ↑ Pierce R C, Kumaresan V (2006). "The Mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse?". Neuroscience & Biobehavioral Reviews. 30 (2): 215–238. doi:10.1016/j.neubiorev.2005.04.016. PMID 16099045.
- ↑ Ikemoto S (2010). "Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory". Neurosci Biobehav Rev. 35 (2): 129–50. doi:10.1016/j.neubiorev.2010.02.001. PMC 2894302 . PMID 20149820.