
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and may require a mordant to improve the fastness of the dye on the fiber.

Indigo dye is an organic compound with a distinctive blue color. Indigo is a natural dye extracted from the leaves of some plants of the Indigofera genus, in particular Indigofera tinctoria; dye-bearing Indigofera plants were commonly grown and used throughout the world, in Asia in particular, as an important crop, with the production of indigo dyestuff economically important due to the historical rarity of other blue dyestuffs.

Haematoxylin or hematoxylin, also called natural black 1 or C.I. 75290, is a compound extracted from heartwood of the logwood tree with a chemical formula of C
16H
14O
6. This naturally derived dye has been used as a histologic stain, as an ink and as a dye in the textile and leather industry. As a dye, haematoxylin has been called palo de Campeche, logwood extract, bluewood and blackwood. In histology, haematoxylin staining is commonly followed by counterstaining with eosin. When paired, this staining procedure is known as H&E staining and is one of the most commonly used combinations in histology. In addition to its use in the H&E stain, haematoxylin is also a component of the Papanicolaou stain which is widely used in the study of cytology specimens.

A mordant or dye fixative is a substance used to set dyes on fabrics. It does this by forming a coordination complex with the dye, which then attaches to the fabric. It may be used for dyeing fabrics or for intensifying stains in cell or tissue preparations. Although mordants are still used, especially by small batch dyers, it has been largely displaced in industry by directs.
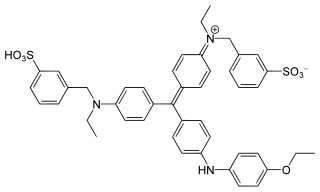
Coomassie brilliant blue is the name of two similar triphenylmethane dyes that were developed for use in the textile industry but are now commonly used for staining proteins in analytical biochemistry. Coomassie brilliant blue G-250 differs from Coomassie brilliant blue R-250 by the addition of two methyl groups. The name "Coomassie" is a registered trademark of Imperial Chemical Industries.

Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triarylmethane dye used as a histological stain and in Gram's method of classifying bacteria. Crystal violet has antibacterial, antifungal, and anthelmintic (vermicide) properties and was formerly important as a topical antiseptic. The medical use of the dye has been largely superseded by more modern drugs, although it is still listed by the World Health Organization.

An acid dye is a dye that is typically applied to a textile at low pH. They are mainly used to dye wool, not cotton fabrics. Some acid dyes are used as food colorants, and some can also be used to stain organelles in the medical field.
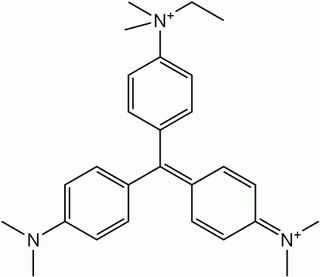
The dye Ethyl Green (C.I. 42590; C27H35BrClN3) is a triarylmethane dye. It is soluble in water.

Eosin Y, also called C.I. 45380 or C.I. Acid Red 87, is a member of the triarylmethane dyes. It is produced from fluorescein by bromination.
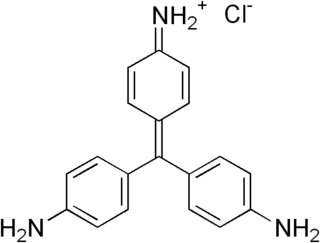
Pararosaniline, Basic Red 9, or C.I. 42500 is an organic compound with the formula [(H2NC6H4)3C]Cl. It is a magenta solid with a variety of uses as a dye. It is one of the four components of basic fuchsine. (The others are rosaniline, new fuchsine and magenta II.) It is structurally related to other triarylmethane dyes called methyl violets including crystal violet, which feature methyl groups on nitrogen.

Orcein, also archil, orchil, lacmus and C.I. Natural Red 28, are names for dyes extracted from several species of lichen, commonly known as "orchella weeds", found in various parts of the world. A major source is the archil lichen, Roccella tinctoria. Orcinol is extracted from such lichens. It is then converted to orcein by ammonia and air. In traditional dye-making methods, urine was used as the ammonia source. If the conversion is carried out in the presence of potassium carbonate, calcium hydroxide, and calcium sulfate, the result is litmus, a more complex molecule. The manufacture was described by Cocq in 1812 and in the UK in 1874. Edmund Roberts noted orchilla as a principal export of the Cape Verde islands, superior to the same kind of "moss" found in Italy or the Canary Islands, that in 1832 was yielding an annual revenue of $200,000. Commercial archil is either a powder or a paste. It is red in acidic pH and blue in alkaline pH.

Light green SF, also called C.I. 42095, light green SF yellowish, is a green triarylmethane dye.
Bismarck brown Y also called C.I. 21000 and C.I. Basic Brown 1, is a diazo dye with the idealized formula [(H2N)2C6H3N2]2C6H4. The dye is a mixture of closely related compounds. It was one of the earliest azo dyes, being described in 1863 by German chemist Carl Alexander von Martius. It is used in histology for staining tissues.

Colored smoke is a kind of smoke created by an aerosol of small particles of a suitable pigment or dye.

Solvent Violet 13, also known as D&C Violet No.2, oil violet, Solvent Blue 90, Alizarine Violet 3B, Alizurol Purple, Duranol Brilliant Violet TG, Ahcoquinone Blue IR base, Quinizarin Blue, Disperse Blue 72, and C.I. 60725, is a synthetic anthraquinone dye with bright bluish violet hue. It is a solid insoluble in water and soluble in acetone, toluene, and benzene. Its chemical formula is C21H15NO3, and its structure is 1-hydroxy-4-(p-tolylamino)anthraquinone, or 1-hydroxy-4-[(4-methylphenyl)amino]-9,10-anthracenedione or 1-hydroxy-4-(4-methylanilino)anthraquinone.

Fountain pen ink is a water-based ink intended for use with fountain pens.
Triarylmethane dyes are synthetic organic compounds containing triphenylmethane backbones. As dyes, these compounds are intensely colored. They are produced industrially as dyes.
Stain removal is the process of removing a mark or spot left by one substance on a specific surface like a fabric. A solvent or detergent is generally used to conduct stain removal and many of these are available over the counter.

Alcian blue is any member of a family of polyvalent basic dyes, of which the Alcian blue 8G has been historically the most common and the most reliable member. It is used to stain acidic polysaccharides such as glycosaminoglycans in cartilages and other body structures, some types of mucopolysaccharides, sialylated glycocalyx of cells etc. For many of these targets it is one of the most widely used cationic dyes for both light and electron microscopy. Use of alcian blue has historically been a popular staining method in histology especially for light microscopy in paraffin embedded sections and in semithin resin sections. The tissue parts that specifically stain by this dye become blue to bluish-green after staining and are called "Alcianophilic". Alcian blue staining can be combined with H&E staining, PAS staining and van Gieson staining methods. Alcian blue can be used to quantitate acidic glycans both in microspectrophotometric quantitation in solution or for staining glycoproteins in polyacrylamide gels or on western blots. Biochemists had used it to assay acid polysaccharides in urine since the 1960s for diagnosis of diseases like mucopolysaccharidosis but from 1970's, partly due to lack of availability of Alcian and partly due to length and tediousness of the procedure, alternative methods had to be developed e.g. Dimethyl methylene blue method.

Acid Blue 25 (C20H13N2NaO5S) is an acid dye that is water-soluble and anionic and used for adsorption research. The structure is an anthraquinone.





















