Related Research Articles
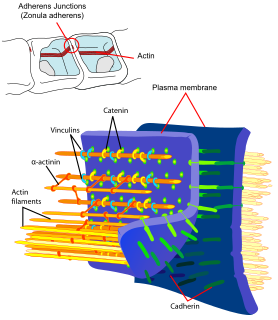
Cadherins (named for "calcium-dependent adhesion") are a type of cell adhesion molecule (CAM) that are important in the formation of adherens junctions to allow cells to adhere to each other. Cadherins are a class of type-1 transmembrane proteins, and they are dependent on calcium (Ca2+) ions to function, hence their name. Cell-cell adhesion is mediated by extracellular cadherin domains, whereas the intracellular cytoplasmic tail associates with numerous adaptors and signaling proteins, collectively referred to as the cadherin adhesome.

Catenins are a family of proteins found in complexes with cadherin cell adhesion molecules of animal cells. The first two catenins that were identified became known as α-catenin and β-catenin. α-Catenin can bind to β-catenin and can also bind filamentous actin (F-actin). β-Catenin binds directly to the cytoplasmic tail of classical cadherins. Additional catenins such as γ-catenin and δ-catenin have been identified. The name "catenin" was originally selected because it was suspected that catenins might link cadherins to the cytoskeleton.

In mammalian cells, vinculin is a membrane-cytoskeletal protein in focal adhesion plaques that is involved in linkage of integrin adhesion molecules to the actin cytoskeleton. Vinculin is a cytoskeletal protein associated with cell-cell and cell-matrix junctions, where it is thought to function as one of several interacting proteins involved in anchoring F-actin to the membrane.

Adherens junctions are protein complexes that occur at cell–cell junctions, cell–matrix junctions in epithelial and endothelial tissues, usually more basal than tight junctions. An adherens junction is defined as a cell junction whose cytoplasmic face is linked to the actin cytoskeleton. They can appear as bands encircling the cell or as spots of attachment to the extracellular matrix . Adherens junctions uniquely disassemble in uterine epithelial cells to allow the blastocyst to penetrate between epithelial cells.
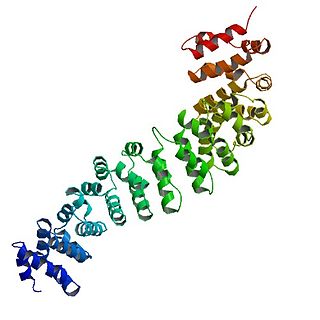
Catenin beta-1, also known as beta-catenin (β-catenin), is a protein that in humans is encoded by the CTNNB1 gene.
An armadillo repeat is the name of a characteristic, repetitive amino acid sequence of about 40 residues in length that is found in many proteins. Proteins that contain armadillo repeats typically contain several tandemly repeated copies. Each armadillo repeat is composed of a pair of alpha helices that form a hairpin structure. Multiple copies of the repeat form what is known as an alpha solenoid structure.

ENA/VASP homology proteins or EVH proteins are a family of closely related proteins involved in cell motility in vertebrate and invertebrate animals. EVH proteins are modular proteins that are involved in actin polymerization, as well as interactions with other proteins. Within the cell, Ena/VASP proteins are found at the leading edge of Lamellipodia and at the tips of filopodia. Ena, the founding member of the family was discovered in a drosophila genetic screen for mutations that act as dominant suppressors of the abl non receptor tyrosine kinase. Invertebrate animals have one Ena homologue, whereas mammals have three, named Mena, VASP, and Evl.
Talin is a high-molecular-weight cytoskeletal protein concentrated at regions of cell–substratum contact and, in lymphocytes, at cell–cell contacts. Discovered in 1983 by Keith Burridge and colleagues, talin is a ubiquitous cytosolic protein that is found in high concentrations in focal adhesions. It is capable of linking integrins to the actin cytoskeleton either directly or indirectly by interacting with vinculin and α-actinin.

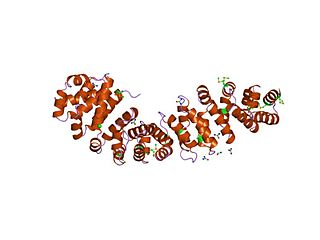
Alpha-catenin functions as the primary protein link between cadherins and the actin cytoskeleton. It has been reported that the actin binding proteins vinculin and alpha-actinin can bind to alpha-catenin. It has been suggested that alpha-catenin does not bind with high affinity to both actin filaments and the E-cadherin-beta-catenin complex at the same time. It has been observed that when alpha-catenin is not in a molecular complex with beta-catenin, it dimerizes and functions to regulate actin filament assembly, possibly by competing with Arp2/3 protein. Alpha catenin exhibits significant protein dynamics. However, a protein complex including a cadherin, actin, beta-catenin and alpha-catenin has not been isolated.
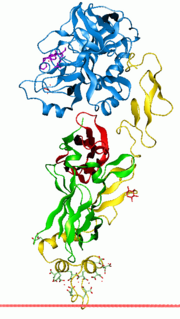
Vitamin K-dependent carboxylation/gamma-carboxyglutamic (GLA) domain is a protein domain that contains post-translational modifications of many glutamate residues by vitamin K-dependent carboxylation to form γ-carboxyglutamate (Gla). Proteins with this domain are known informally as Gla proteins. The Gla residues are responsible for the high-affinity binding of calcium ions.

N-cadherin, also known as Cadherin-2 (CDH2) or neural cadherin (NCAD) is a protein that in humans is encoded by the CDH2 gene. CDH2 has also been designated as CD325. N-cadherin is a transmembrane protein expressed in multiple tissues and functions to mediate cell–cell adhesion. In cardiac muscle, N-cadherin is an integral component in adherens junctions residing at intercalated discs, which function to mechanically and electrically couple adjacent cardiomyocytes. Alterations in expression and integrity of N-cadherin protein has been observed in various forms of disease, including human dilated cardiomyopathy. Variants in CDH2 have also been identified to cause a syndromic neurodevelopmental disorder.

Cadherin-3, also known as P-Cadherin, is a protein that in humans is encoded by the CDH3 gene.

Cadherin-1 also known as CAM 120/80 or epithelial cadherin (E-cadherin) or uvomorulin is a protein that in humans is encoded by the CDH1 gene. Mutations are correlated with gastric, breast, colorectal, thyroid, and ovarian cancers. CDH1 has also been designated as CD324. It is a tumor suppressor gene.

αE-catenin, also known as Catenin alpha-1 is a protein that in humans is encoded by the CTNNA1 gene. αE-catenin is highly expressed in cardiac muscle and localizes to adherens junctions at intercalated disc structures where it functions to mediate the anchorage of actin filaments to the sarcolemma. αE-catenin also plays a role in tumor metastasis and skin cell function.
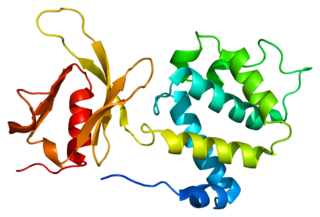
Talin-1 is a protein that in humans is encoded by the TLN1 gene. Talin-1 is ubiquitously expressed, and is localized to costamere structures in cardiac and skeletal muscle cells, and to focal adhesions in smooth muscle and non-muscle cells. Talin-1 functions to mediate cell-cell adhesion via the linkage of integrins to the actin cytoskeleton and in the activation of integrins. Altered expression of talin-1 has been observed in patients with heart failure, however no mutations in TLN1 have been linked with specific diseases.
WH1 domain is an evolutionary conserved protein domain. Therefore, it has an important function.
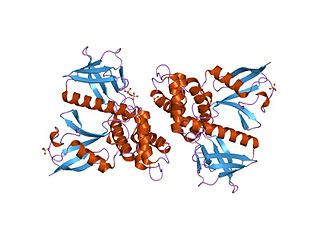
In molecular biology, the FERM domain is a widespread protein module involved in localising proteins to the plasma membrane. FERM domains are found in a number of cytoskeletal-associated proteins that associate with various proteins at the interface between the plasma membrane and the cytoskeleton. The FERM domain is located at the N terminus in the majority of proteins in which it is found.

In structural and cell biology, the focal adhesion targeting domain is a conserved protein domain that was first identified in focal adhesion kinase (FAK), also known as PTK2 protein tyrosine kinase 2 (PTK2).

In molecular biology, the GYF domain is an approximately 60-amino acid protein domain which contains a conserved GP[YF]xxxx[MV]xxWxxx[GN]YF motif. It was identified in the human intracellular protein termed CD2 binding protein 2 (CD2BP2), which binds to a site containing two tandem PPPGHR segments within the cytoplasmic region of CD2. Binding experiments and mutational analyses have demonstrated the critical importance of the GYF tripeptide in ligand binding. A GYF domain is also found in several other eukaryotic proteins of unknown function. It has been proposed that the GYF domain found in these proteins could also be involved in proline-rich sequence recognition. Resolution of the structure of the CD2BP2 GYF domain by NMR spectroscopy revealed a compact domain with a beta-beta-alpha-beta-beta topology, where the single alpha-helix is tilted away from the twisted, anti-parallel beta-sheet. The conserved residues of the GYF domain create a contiguous patch of predominantly hydrophobic nature which forms an integral part of the ligand-binding site. There is limited homology within the C-terminal 20-30 amino acids of various GYF domains, supporting the idea that this part of the domain is structurally but not functionally important.
Long-term potentiation (LTP), thought to be the cellular basis for learning and memory, involves a specific signal transmission process that underlies synaptic plasticity. Among the many mechanisms responsible for the maintenance of synaptic plasticity is the cadherin–catenin complex. By forming complexes with intracellular catenin proteins, neural cadherins (N-cadherins) serve as a link between synaptic activity and synaptic plasticity, and play important roles in the processes of learning and memory.
References
- ↑ Otto JJ (1990). "Vinculin". Cell Motil. Cytoskeleton . 16 (1): 1–6. doi:10.1002/cm.970160102. PMID 2112986.
- ↑ Lottspeich F, Eckerskorn C, Herrenknecht K, Ozawa M, Lenter M, Kemler R (1991). "The uvomorulin-anchorage protein alpha catenin is a vinculin homologue". Proc. Natl. Acad. Sci. U.S.A. 88 (20): 9156–9160. Bibcode:1991PNAS...88.9156H. doi: 10.1073/pnas.88.20.9156 . PMC 52671 . PMID 1924379.