
Glass is an amorphous (non-crystalline) solid. Because it is often transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window panes, tableware, and optics. Some common objects made of glass like "a glass" of water, "glasses", and "magnifying glass", are named after the material.

Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which increases the substance's temperature to the melting point. At the melting point, the ordering of ions or molecules in the solid breaks down to a less ordered state, and the solid melts to become a liquid.
Rheology is the study of the flow of matter, primarily in a fluid state but also as "soft solids" or solids under conditions in which they respond with plastic flow rather than deforming elastically in response to an applied force. Rheology is the branch of physics that deals with the deformation and flow of materials, both solids and liquids.

The melting point of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa.

Differential scanning calorimetry (DSC) is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and reference are maintained at nearly the same temperature throughout the experiment.

Freezing is a phase transition in which a liquid turns into a solid when its temperature is lowered below its freezing point.

Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid below its freezing point without it becoming a solid. As per the established international definition, supercooling means ‘cooling a substance below the normal freezing point without solidification’ While it can be achieved by different physical means, the postponed solidification is most often due to the absence of seed crystals or nuclei around which a crystal structure can form. The supercooling of water can be achieved without any special techniques other than chemical demineralization, down to −48.3 °C (−54.9 °F). Supercooled water can occur naturally, for example in the atmosphere, animals or plants.

An amorphous metal is a solid metallic material, usually an alloy, with disordered atomic-scale structure. Most metals are crystalline in their solid state, which means they have a highly ordered arrangement of atoms. Amorphous metals are non-crystalline, and have a glass-like structure. But unlike common glasses, such as window glass, which are typically electrical insulators, amorphous metals have good electrical conductivity and can show metallic luster.

Polyamorphism is the ability of a substance to exist in several different amorphous modifications. It is analogous to the polymorphism of crystalline materials. Many amorphous substances can exist with different amorphous characteristics. However, polyamorphism requires two distinct amorphous states with a clear, discontinuous (first-order) phase transition between them. When such a transition occurs between two stable liquid states, a polyamorphic transition may also be referred to as a liquid–liquid phase transition.

Soda–lime glass, also called soda–lime–silica glass, is the most prevalent type of glass, used for windowpanes and glass containers for beverages, food, and some commodity items. Some glass bakeware is made of soda-lime glass, as opposed to the more common borosilicate glass. Soda–lime glass accounts for about 90% of manufactured glass.

David Turnbull was an American physical chemist who worked in the interdisciplinary fields of materials science and applied physics. Turnbull made seminal contributions to solidification theory and glass formation. Turnbull was born in Elmira, Elmira Township, Stark County, Illinois. He graduated from high school in 1932 and then received a bachelor's degree in 1936 from Monmouth College (Illinois), specializing in physical chemistry. He received his Ph.D. in physical chemistry under Thomas Erwin Phipps from the University of Illinois in 1939. He was on the faculty of Case Institute of Technology from 1939 to 1946 before eventually becoming a professor at Harvard University.
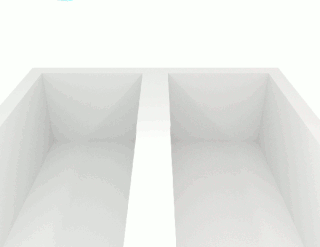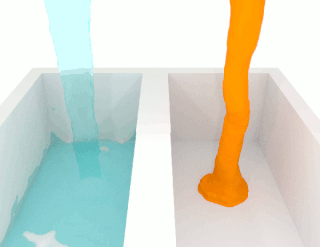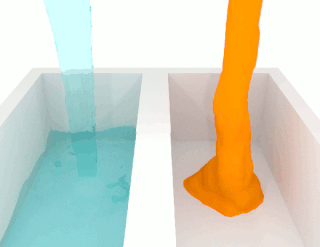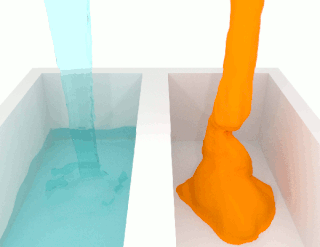
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water. Viscosity is defined scientifically as a force multiplied by a time divided by an area. Thus its SI units are newton-seconds per square meter, or pascal-seconds.
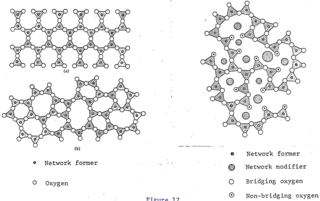
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification.
Johari–Goldstein relaxation, also known as the JG β-relaxation, is a universal property of glasses and certain other disordered materials. Proposed in 1969 by Martin Goldstein, JG β-relaxation were described as a secondary relaxation mechanism required to explain the viscosity behavior of liquids approaching the glass transition in the potential energy landscape picture presented in Goldstein's seminal 1969 paper. Previous experiments on glass forming liquids showed multiple relaxation times present in liquids measured by time dependent compliance measurements. Gyan Johari and Martin Goldstein measured the dielectric loss spectrum of a set of rigid glass forming molecules to further test the hypothesis of Goldstein in 1969. The relaxation, a peak in mechanical or dielectric loss at a particular frequency, had previously been attributed to a type of molecular flexibility. The fact that such a loss peak shows up in glasses of rigid molecules lacking this flexibility demonstrated its universal character.
Wolfgang Götze was a German theoretical physicist.
Charles Austen Angell was a renowned Australian and American physical chemist known for his prolific and highly cited research on the chemistry and physics of glasses and glass-forming liquids. He was internationally recognized as a luminary in the fields of glasses, liquids, water and ionic liquids.

A liquid metal is a metal or a metal alloy which is liquid at or near room temperature.
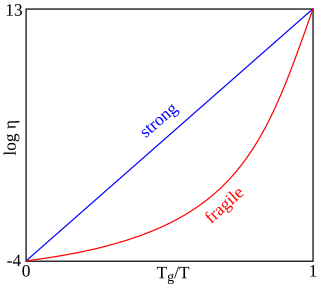
In glass sciences, fragility or "kinetic fragility" is a concept proposed by the Australian-American physical chemist C. Austen Angell. Fragility characterizes how rapidly the viscosity of a glass forming liquid approaches a very large value approximately 1012 Pa s during cooling. At this viscosity, the liquid is "frozen" into a solid and the corresponding temperature is known as the glass transition temperature Tg. Materials with a higher fragility have a more rapid increase in viscosity as approaching Tg, while those with a lower fragility have a slower increase in viscosity. Fragility is one of the most important concepts to understand viscous liquids and glasses. Fragility may be related to the presence of dynamical heterogeneity in glass forming liquids, as well as to the breakdown of the usual Stokes–Einstein relationship between viscosity and diffusion. Fragility has no direct relationship with the colloquial meaning of the word "fragility", which more closely relates to the brittleness of a material.
Rheological weldability (RW) of thermoplastics considers the materials flow characteristics in determining the weldability of the given material. The process of welding thermal plastics requires three general steps, first is surface preparation. The second step is the application of heat and pressure to create intimate contact between the components being joined and initiate inter-molecular diffusion across the joint and the third step is cooling. RW can be used to determine the effectiveness of the second step of the process for given materials.
Alessio Zaccone is an Italian physicist.














