Related Research Articles

A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and may require a mordant to improve the fastness of the dye on the fiber.

Histology, also known as microscopic anatomy or microanatomy, is the branch of biology that studies the microscopic anatomy of biological tissues. Histology is the microscopic counterpart to gross anatomy, which looks at larger structures visible without a microscope. Although one may divide microscopic anatomy into organology, the study of organs, histology, the study of tissues, and cytology, the study of cells, modern usage places all of these topics under the field of histology. In medicine, histopathology is the branch of histology that includes the microscopic identification and study of diseased tissue. In the field of paleontology, the term paleohistology refers to the histology of fossil organisms.

Haematoxylin or hematoxylin, also called natural black 1 or C.I. 75290, is a compound extracted from heartwood of the logwood tree with a chemical formula of C
16H
14O
6. This naturally derived dye has been used as a histologic stain, as an ink and as a dye in the textile and leather industry. As a dye, haematoxylin has been called palo de Campeche, logwood extract, bluewood and blackwood. In histology, haematoxylin staining is commonly followed by counterstaining with eosin. When paired, this staining procedure is known as H&E staining and is one of the most commonly used combinations in histology. In addition to its use in the H&E stain, haematoxylin is also a component of the Papanicolaou stain which is widely used in the study of cytology specimens.

Eosin is the name of several fluorescent acidic compounds which bind to and form salts with basic, or eosinophilic, compounds like proteins containing amino acid residues such as arginine and lysine, and stains them dark red or pink as a result of the actions of bromine on eosin. In addition to staining proteins in the cytoplasm, it can be used to stain collagen and muscle fibers for examination under the microscope. Structures that stain readily with eosin are termed eosinophilic. In the field of histology, Eosin Y is the form of eosin used most often as a histologic stain.

Romanowsky staining, also known as Romanowsky–Giemsa staining, is a prototypical staining technique that was the forerunner of several distinct but similar stains widely used in hematology and cytopathology. Romanowsky-type stains are used to differentiate cells for microscopic examination in pathological specimens, especially blood and bone marrow films, and to detect parasites such as malaria within the blood. Stains that are related to or derived from the Romanowsky-type stains include Giemsa, Jenner, Wright, Field, May–Grünwald and Leishman stains. The staining technique is named after the Russian physician Dmitri Leonidovich Romanowsky (1861–1921), who was one of the first to recognize its potential for use as a blood stain.
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are an immune cell or some types of venom, e.g. from the puff adder or brown recluse spider.

Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology, in cytology, and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues, cell populations, or organelles within individual cells.

Wright's stain is a hematologic stain that facilitates the differentiation of blood cell types. It is classically a mixture of eosin (red) and methylene blue dyes. It is used primarily to stain peripheral blood smears, urine samples, and bone marrow aspirates, which are examined under a light microscope. In cytogenetics, it is used to stain chromosomes to facilitate diagnosis of syndromes and diseases.

Giemsa stain, named after German chemist and bacteriologist Gustav Giemsa, is a nucleic acid stain used in cytogenetics and for the histopathological diagnosis of malaria and other parasites.

Basophilic is a technical term used by pathologists. It describes the appearance of cells, tissues and cellular structures as seen through the microscope after a histological section has been stained with a basic dye. The most common such dye is haematoxylin.

Hoechst stains are part of a family of blue fluorescent dyes used to stain DNA. These bis-benzimides were originally developed by Hoechst AG, which numbered all their compounds so that the dye Hoechst 33342 is the 33,342nd compound made by the company. There are three related Hoechst stains: Hoechst 33258, Hoechst 33342, and Hoechst 34580. The dyes Hoechst 33258 and Hoechst 33342 are the ones most commonly used and they have similar excitation–emission spectra.

An acid dye is a dye that is typically applied to a textile at low pH. They are mainly used to dye wool, not cotton fabrics. Some acid dyes are used as food colorants, and some can also be used to stain organelles in the medical field.
Trypan blue is an azo dye. It is a direct dye for cotton textiles. In biosciences, it is used as a vital stain to selectively colour dead tissues or cells blue.

Papanicolaou stain is a multichromatic (multicolored) cytological staining technique developed by George Papanicolaou in 1942. The Papanicolaou stain is one of the most widely used stains in cytology, where it is used to aid pathologists in making a diagnosis. Although most notable for its use in the detection of cervical cancer in the Pap test or Pap smear, it is also used to stain non-gynecological specimen preparations from a variety of bodily secretions and from small needle biopsies of organs and tissues. Papanicolaou published three formulations of this stain in 1942, 1954, and 1960.

Hematoxylin and eosin stain is one of the principal tissue stains used in histology. It is the most widely used stain in medical diagnosis and is often the gold standard. For example, when a pathologist looks at a biopsy of a suspected cancer, the histological section is likely to be stained with H&E.

Neutral red is a eurhodin dye used for staining in histology. It stains lysosomes red. It is used as a general stain in histology, as a counterstain in combination with other dyes, and for many staining methods. Together with Janus Green B, it is used to stain embryonal tissues and supravital staining of blood. Can be used for staining Golgi apparatus in cells and Nissl granules in neurons.
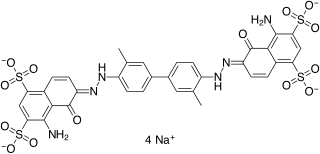
T-1824 or Evans blue, often incorrectly rendered as Evan's blue, is an azo dye that has a very high affinity for serum albumin. Because of this, it can be useful in physiology in estimating the proportion of body water contained in blood plasma. It fluoresces with excitation peaks at 470 and 540 nm and an emission peak at 680 nm.
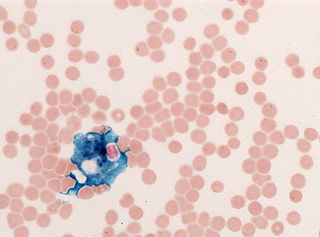
In histology, histopathology, and clinical pathology, Perls Prussian blue is a commonly used method to detect the presence of iron in tissue or cell samples. Perls Prussian Blue derives its name from the German pathologist Max Perls (1843–1881), who described the technique in 1867. The method does not involve the application of a dye, but rather causes the pigment Prussian blue to form directly within the tissue. The method stains mostly iron in the ferric state which includes ferritin and hemosiderin, rather than iron in the ferrous state.

A viability assay is an assay that is created to determine the ability of organs, cells or tissues to maintain or recover a state of survival. Viability can be distinguished from the all-or-nothing states of life and death by the use of a quantifiable index that ranges between the integers of 0 and 1 or, if more easily understood, the range of 0% and 100%. Viability can be observed through the physical properties of cells, tissues, and organs. Some of these include mechanical activity, motility, such as with spermatozoa and granulocytes, the contraction of muscle tissue or cells, mitotic activity in cellular functions, and more. Viability assays provide a more precise basis for measurement of an organism's level of vitality.

Supravital staining is a method of staining used in microscopy to examine living cells that have been removed from an organism. It differs from intravital staining, which is done by injecting or otherwise introducing the stain into the body. Thus a supravital stain may have a greater toxicity, as only a few cells need to survive it a short while. The term "vital stain" is used by some authors to refer specifically to an intravital stain, and by others interchangeably with a supravital stain, the core concept being that the cell being examined is still alive. As the cells are alive and unfixed, outside the body, supravital stains are temporary in nature.
References
- ↑ Rodrigues EB, Costa EF, Penha FM, Melo GB, Bottós J, Dib E, Furlani B, Lima VC, Maia M, Meyer CH, Höfling-Lima AL, Farah ME (2009). "The use of vital dyes in ocular surgery". Survey of Ophthalmology. 54 (5): 576–617. doi:10.1016/j.survophthal.2009.04.011. PMID 19682624.
- ↑ Hathaway WE, Newby LA, Githens JH (1964). "The Acridine Orange Viability Test Applied to Bone Marrow Cells. I. Correlation with Trypan Blue and Eosin Dye Exclusion and Tissue Culture Transformation". Blood. 23 (4): 517–525. doi: 10.1182/blood.V23.4.517.517 . PMID 14138242.
- ↑ Keren DF (2001). "Section X: Immunopathology". In McClatchey KD (ed.). Clinical Laboratory Medicine (2nd ed.). Lippincott Williams & Wilkins. p. 1384. ISBN 0-683-30751-7.
- ↑ Lecoeur H (2002). "Nuclear apoptosis detection by flow cytometry: influence of endogenous endonucleases". Exp. Cell Res. 277 (1): 1–14. doi:10.1006/excr.2002.5537. PMID 12061813.