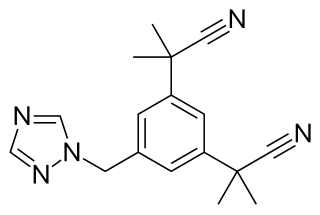
Anastrozole, sold under the brand name Arimidex among others, is an antiestrogenic medication used in addition to other treatments for breast cancer. Specifically it is used for hormone receptor-positive breast cancer. It has also been used to prevent breast cancer in those at high risk. It is taken by mouth.

An imidazopyridine is a nitrogen containing heterocycle that is also a class of drugs that contain this same chemical substructure. In general, they are GABAA receptor agonists, however recently proton pump inhibitors, aromatase inhibitors, NSAIDs and other classes of drugs in this class have been developed as well. Despite usually being similar to them in effect, they are not chemically related to benzodiazepines. As such, GABAA-agonizing imidazopyridines, pyrazolopyrimidines, and cyclopyrrones are sometimes grouped together and referred to as "nonbenzodiazepines." Imidazopyridines include:

Aromatase, also called estrogen synthetase or estrogen synthase, is an enzyme responsible for a key step in the biosynthesis of estrogens. It is CYP19A1, a member of the cytochrome P450 superfamily, which are monooxygenases that catalyze many reactions involved in steroidogenesis. In particular, aromatase is responsible for the aromatization of androgens into estrogens. The enzyme aromatase can be found in many tissues including gonads, brain, adipose tissue, placenta, blood vessels, skin, and bone, as well as in tissue of endometriosis, uterine fibroids, breast cancer, and endometrial cancer. It is an important factor in sexual development.

Aromatase inhibitors (AIs) are a class of drugs used in the treatment of breast cancer in postmenopausal women and in men, and gynecomastia in men. They may also be used off-label to reduce estrogen conversion when supplementing testosterone exogenously. They may also be used for chemoprevention in women at high risk for breast cancer.

Aminoglutethimide (AG), sold under the brand names Elipten, Cytadren, and Orimeten among others, is a medication which has been used in the treatment of seizures, Cushing's syndrome, breast cancer, and prostate cancer, among other indications. It has also been used by bodybuilders, athletes, and other men for muscle-building and performance- and physique-enhancing purposes. AG is taken by mouth three or four times per day.
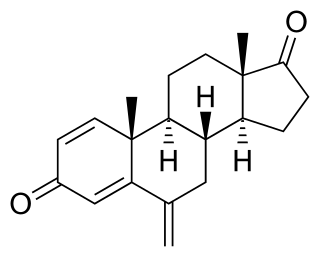
Exemestane, sold under the brand name Aromasin among others, is a medication used to treat breast cancer. It is a member of the class of antiestrogens known as aromatase inhibitors. Some breast cancers require estrogen to grow. Those cancers have estrogen receptors (ERs), and are called ER-positive. They may also be called estrogen-responsive, hormonally-responsive, or hormone-receptor-positive. Aromatase is an enzyme that synthesizes estrogen. Aromatase inhibitors block the synthesis of estrogen. This lowers the estrogen level, and slows the growth of cancers.
A nonsteroidal compound is a drug that is not a steroid nor a steroid derivative. Nonsteroidal anti-inflammatory drugs (NSAIDs) are distinguished from corticosteroids as a class of anti-inflammatory agents.

Chrysin, also called 5,7-dihydroxyflavone, is a flavone found in honey, propolis, the passion flowers, Passiflora caerulea and Passiflora incarnata, and in Oroxylum indicum. It is extracted from various plants, such as the blue passion flower. Following oral intake by humans, chrysin has low bioavailability and rapid excretion. It is under basic research to evaluate its safety and potential biological effects.

Lasofoxifene, sold under the brand name Fablyn, is a nonsteroidal selective estrogen receptor modulator (SERM) which is marketed by Pfizer in Lithuania and Portugal for the prevention and treatment of osteoporosis and for the treatment of vaginal atrophy, and the result of an exclusive research collaboration with Ligand Pharmaceuticals (LGND). It also appears to have had a statistically significant effect of reducing breast cancer in women according to a study published in The Journal of the National Cancer Institute.

1,4,6-Androstatriene-3,17-dione (ATD) is a potent irreversible aromatase inhibitor that inhibits estrogen biosynthesis by permanently binding and inactivating aromatase in adipose and peripheral tissue. It is used to control estrogen synthesis.
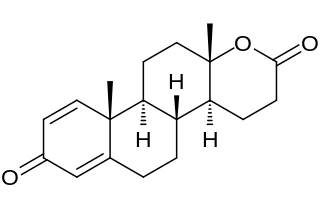
Testolactone is a non-selective, irreversible, steroidal aromatase inhibitor which is used as an antineoplastic drug to treat advanced-stage breast cancer. The drug was discontinued in 2008 and is no longer available for medical use.
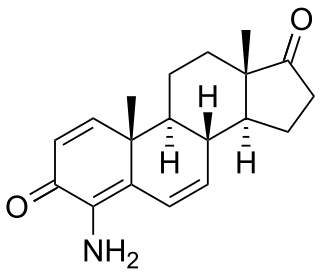
Minamestane (INN is a steroidal aromatase inhibitor which was under development by Farmitalia-Carlo Erba as an antineoplastic agent in the mid-1990s but was never marketed.
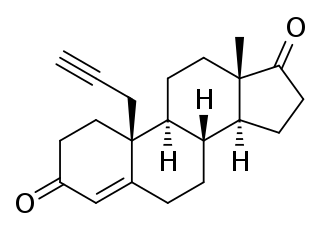
Plomestane is a steroidal, irreversible aromatase inhibitor which was under development by Marion Merrell Dow/Hoechst Marion Russell as an antineoplastic agent for the treatment of breast cancer. It was found to be effective in preclinical studies and was also found to produce few adverse effects in human clinical trials, significantly reducing estrogen levels with a single administration. However, development of the drug for clinical use was halted due to "technical issues" and it was never marketed.
Steroidal aromatase inhibitors are a class of drugs that are mostly used for treating breast cancer in postmenopausal women. High levels of estrogen in breast tissue increases the risk of developing breast cancer and the enzyme aromatase is considered to be a good therapeutic target when treating breast cancer due to it being involved in the final step of estrogen biosynthetic pathway and also its inhibition will not affect production of other steroids. Aromatase Inhibitors are classified into two categories based on their structure, nonsteroidal and steroidal; the latter resemble the structure of androstenedione. Steroidal aromatase inhibitors irreversibly inhibit the enzyme by binding covalently to the binding site of aromatase so the substrate cannot access it.

Osimertinib, sold under the brand name Tagrisso, is a medication used to treat non-small-cell lung carcinomas with specific mutations. It is a third-generation epidermal growth factor receptor tyrosine kinase inhibitor.
A selective estrogen receptor degrader or downregulator (SERD) is a type of drug that selectively binds to the estrogen receptor (ER) and induces its degradation, and thus causes its downregulation. SERDs are used in the treatment of estrogen receptor-positive breast cancer, particularly in cases where tumors have developed resistance to other forms of endocrine therapy, such as selective estrogen receptor modulators (SERMs) or aromatase inhibitors.

Angela Hartley Brodie was a British biochemist who pioneered development of steroidal aromatase inhibitors in cancer research. Born in Lancashire, Brodie studied chemical pathology to a doctoral level in Sheffield and was awarded a fellowship sponsored by National Institutes of Health. After 17 years of working in Shrewsbury, Massachusetts on oral contraceptives with Harry Brodie, whom she married, she switched focus to the effects of the oestrogen-producing enzyme, aromatase, on breast cancer.

Ribociclib, sold under the brand name Kisqali, is a medication used for the treatment of certain kinds of breast cancer. Ribociclib is a kinase inhibitor. It was developed by Novartis and Astex Pharmaceuticals.

Irosustat is an orally active, irreversible, nonsteroidal inhibitor of steroid sulfatase (STS) and member of the aryl sulfamate ester class of drugs that was under development by Sterix Ltd and Ipsen for the treatment of hormone-sensitive cancers such as breast cancer, prostate cancer, and endometrial cancer but has not yet been marketed. The drug was first designed and synthesized in the group of Professor Barry V L Potter at the Department of Pharmacy & Pharmacology, University of Bath, working together with Professor Michael J. Reed at Imperial College, London and its initial development was undertaken through the university spin-out company Sterix Ltd and overseen by Cancer Research UK (CRUK). Results of the "first-in-class" clinical trial in breast cancer of an STS inhibitor in humans were published in 2006 and dose optimisation studies and further clinical data have been reported.

Non-Steroidal Aromatase Inhibitors (NSAIs) are one of two categories of aromatase inhibitors (AIs). AIs are divided into two categories, steroidal aromatase inhibitors and non-steroidal aromatase inhibitors that is based on their mechanism of action and structure. NSAIs are mainly used to treat breast cancer in women. NSAIs binding is a reversible process where NSAIs binds to the aromatase enzyme through non-covalent interactions. When aromatase inhibitors (AIs) are used to treat breast cancer the main target is the aromatase enzyme which is responsible for the high estrogen level.
















