
Anticoagulants, commonly known as blood thinners, are chemical substances that prevent or reduce coagulation of blood, prolonging the clotting time. Some of them occur naturally in blood-eating animals such as leeches and mosquitoes, where they help keep the bite area unclotted long enough for the animal to obtain some blood. As a class of medications, anticoagulants are used in therapy for thrombotic disorders. Oral anticoagulants (OACs) are taken by many people in pill or tablet form, and various intravenous anticoagulant dosage forms are used in hospitals. Some anticoagulants are used in medical equipment, such as sample tubes, blood transfusion bags, heart–lung machines, and dialysis equipment. One of the first anticoagulants, warfarin, was initially approved as a rodenticide.

Venous thrombosis is blockage of a vein caused by a thrombus. A common form of venous thrombosis is deep vein thrombosis (DVT), when a blood clot forms in the deep veins. If a thrombus breaks off (embolizes) and flows to the lungs to lodge there, it becomes a pulmonary embolism (PE), a blood clot in the lungs. The conditions of DVT only, DVT with PE, and PE only, are all captured by the term venous thromboembolism (VTE).

Warfarin is an anticoagulant used as a medication under several brand names including Coumadin, and as a poison for rats and other pests. While the drug is described as a "blood thinner", it does not reduce viscosity but inhibits coagulation, and is commonly used to prevent blood clots in the circulatory system such as deep vein thrombosis and pulmonary embolism, and to protect against stroke in people who have atrial fibrillation, valvular heart disease, or artificial heart valves. Less commonly, it is used following ST-segment elevation myocardial infarction and orthopedic surgery. It is usually taken by mouth, but may also be administered intravenously.
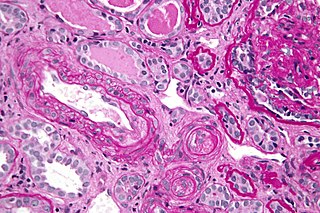
Antiphospholipid syndrome, or antiphospholipid antibody syndrome, is an autoimmune, hypercoagulable state caused by antiphospholipid antibodies. APS provokes blood clots (thrombosis) in both arteries and veins as well as pregnancy-related complications such as miscarriage, stillbirth, preterm delivery, and severe preeclampsia. Although the exact etiology of APS is still not clear, genetics is believed to play a key role in the development of the disease. The diagnostic criteria require one clinical event and two positive blood test results spaced at least three months apart that detect lupus anticoagulant, anti-apolipoprotein antibodies, or anti-cardiolipin antibodies.
Low-molecular-weight heparin (LMWH) is a class of anticoagulant medications. They are used in the prevention of blood clots and treatment of venous thromboembolism and in the treatment of myocardial infarction.

The prothrombin time (PT) – along with its derived measures of prothrombin ratio (PR) and international normalized ratio (INR) – is an assay for evaluating the extrinsic pathway and common pathway of coagulation. This blood test is also called protime INR and PT/INR. They are used to determine the clotting tendency of blood, in such things as the measure of warfarin dosage, liver damage, and vitamin K status. PT measures the following coagulation factors: I (fibrinogen), II (prothrombin), V (proaccelerin), VII (proconvertin), and X.

Heparin-induced thrombocytopenia (HIT) is the development of thrombocytopenia, due to the administration of various forms of heparin, an anticoagulant. HIT predisposes to thrombosis because platelets release microparticles that activate thrombin, thereby leading to thrombosis. When thrombosis is identified the condition is called heparin-induced thrombocytopenia and thrombosis (HITT). HIT is caused by the formation of abnormal antibodies that activate platelets. If someone receiving heparin develops new or worsening thrombosis, or if the platelet count falls, HIT can be confirmed with specific blood tests.

Thrombophilia is an abnormality of blood coagulation that increases the risk of thrombosis. Such abnormalities can be identified in 50% of people who have an episode of thrombosis that was not provoked by other causes. A significant proportion of the population has a detectable thrombophilic abnormality, but most of these develop thrombosis only in the presence of an additional risk factor.
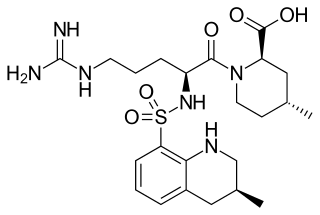
Argatroban is an anticoagulant that is a small molecule direct thrombin inhibitor. In 2000, argatroban was licensed by the Food and Drug Administration (FDA) for prophylaxis or treatment of thrombosis in patients with heparin-induced thrombocytopenia (HIT). In 2002, it was approved for use during percutaneous coronary interventions in patients who have HIT or are at risk for developing it. In 2012, it was approved by the MHRA in the UK for anticoagulation in patients with heparin-induced thrombocytopenia Type II (HIT) who require parenteral antithrombotic therapy.

Fresh frozen plasma (FFP) is a blood product made from the liquid portion of whole blood. It is used to treat conditions in which there are low blood clotting factors or low levels of other blood proteins. It may also be used as the replacement fluid in plasma exchange. Using ABO compatible plasma, while not required, may be recommended. Use as a volume expander is not recommended. It is given by slow injection into a vein.

Hypoprothrombinemia is a rare blood disorder in which a deficiency in immunoreactive prothrombin, produced in the liver, results in an impaired blood clotting reaction, leading to an increased physiological risk for spontaneous bleeding. This condition can be observed in the gastrointestinal system, cranial vault, and superficial integumentary system, affecting both the male and female population. Prothrombin is a critical protein that is involved in the process of hemostasis, as well as illustrating procoagulant activities. This condition is characterized as an autosomal recessive inheritance congenital coagulation disorder affecting 1 per 2,000,000 of the population, worldwide, but is also attributed as acquired.

Protein C deficiency is a rare genetic trait that predisposes to thrombotic disease. It was first described in 1981. The disease belongs to a group of genetic disorders known as thrombophilias. Protein C deficiency is associated with an increased incidence of venous thromboembolism, whereas no association with arterial thrombotic disease has been found.

Phenprocoumon is a long-acting blood thinner drug to be taken by mouth, and a derivative of coumarin. It acts as a vitamin K antagonist and inhibits blood clotting (coagulation) by blocking synthesis of coagulation factors II, VII, IX and X. It is used for the prophylaxis and treatment of thromboembolic disorders such as heart attacks and pulmonary (lung) embolism. The most common adverse effect is bleeding. The drug interacts with a large number of other medications, including aspirin and St John's Wort. It is the standard coumarin used in Germany, Austria, and other European countries.
Prothrombin complex concentrate (PCC), also known as factor IX complex, is a medication made up of blood clotting factors II, IX, and X. Some versions also contain factor VII. It is used to treat and prevent bleeding in hemophilia B if pure factor IX is not available. It may also be used for reversal of warfarin therapy. It is given by slow injection into a vein.
Purpura fulminans is an acute, often fatal, thrombotic disorder which manifests as blood spots, bruising and discolouration of the skin resulting from coagulation in small blood vessels within the skin and rapidly leads to skin necrosis and disseminated intravascular coagulation.
Hypercoagulability in pregnancy is the propensity of pregnant women to develop thrombosis. Pregnancy itself is a factor of hypercoagulability, as a physiologically adaptive mechanism to prevent post partum bleeding. However, when combined with an additional underlying hypercoagulable states, the risk of thrombosis or embolism may become substantial.
Direct factor Xa inhibitors (xabans) are anticoagulants, used to both treat and prevent blood clots in veins, and prevent stroke and embolism in people with atrial fibrillation (AF).

Vitamin K antagonists (VKA) are a group of substances that reduce blood clotting by reducing the action of vitamin K. The term "vitamin K antagonist" is technically a misnomer, as the drugs do not directly antagonise the action of vitamin K in the pharmacological sense, but rather the recycling of vitamin K.
Thromboelastometry (TEM), previously named rotational thromboelastography (ROTEG) or rotational thromboelastometry (ROTEM), is an established viscoelastic method for hemostasis testing in whole blood. It is a modification of traditional thromboelastography (TEG).
Direct thrombin inhibitors (DTIs) are a class of anticoagulant drugs that can be used to prevent and treat embolisms and blood clots caused by various diseases. They inhibit thrombin, a serine protease which affects the coagulation cascade in many ways. DTIs have undergone rapid development since the 90's. With technological advances in genetic engineering the production of recombinant hirudin was made possible which opened the door to this new group of drugs. Before the use of DTIs the therapy and prophylaxis for anticoagulation had stayed the same for over 50 years with the use of heparin derivatives and warfarin which have some well known disadvantages. DTIs are still under development, but the research focus has shifted towards factor Xa inhibitors, or even dual thrombin and fXa inhibitors that have a broader mechanism of action by both inhibiting factor IIa (thrombin) and Xa. A recent review of patents and literature on thrombin inhibitors has demonstrated that the development of allosteric and multi-mechanism inhibitors might lead the way to a safer anticoagulant.














