Related Research Articles

Bicalutamide, sold under the brand name Casodex among others, is an antiandrogen medication that is primarily used to treat prostate cancer. It is typically used together with a gonadotropin-releasing hormone (GnRH) analogue or surgical removal of the testicles to treat metastatic prostate cancer (mPC). To a lesser extent, it is used at high doses for locally advanced prostate cancer (LAPC) as a monotherapy without castration. Bicalutamide was also previously used as monotherapy to treat localized prostate cancer (LPC), but authorization for this use was withdrawn following unfavorable trial findings. Besides prostate cancer, bicalutamide is limitedly used in the treatment of excessive hair growth and scalp hair loss in women, as a puberty blocker and component of feminizing hormone therapy for transgender girls and women, to treat gonadotropin-independent early puberty in boys, and to prevent overly long-lasting erections in men. It is taken by mouth.
5α-Reductase inhibitors (5-ARIs), also known as dihydrotestosterone (DHT) blockers, are a class of medications with antiandrogenic effects which are used primarily in the treatment of enlarged prostate and scalp hair loss. They are also sometimes used to treat excess hair growth in women and as a component of hormone therapy for transgender women.
Nicholas J. Vogelzang was a medical oncologist with Comprehensive Cancer Centers of Nevada (CCCN). He serves as medical director of the Research Executive Committee and Associate Chair of the Developmental Therapeutics and Genitourinary Committees for US Oncology Research. His research interests include clinical trials for genitourinary malignancies and mesothelioma.

A gonadotropin-releasing hormone agonist is a type of medication which affects gonadotropins and sex hormones. They are used for a variety of indications including in fertility medicine and to lower sex hormone levels in the treatment of hormone-sensitive cancers such as prostate cancer and breast cancer, certain gynecological disorders like heavy periods and endometriosis, high testosterone levels in women, early puberty in children, as a part of transgender hormone therapy, and to delay puberty in transgender youth among other uses. It is also used in the suppression of spontaneous ovulation as part of controlled ovarian hyperstimulation, an essential component in IVF. GnRH agonists are given by injections into fat, as implants placed into fat, and as nasal sprays.

Gonadotropin-releasing hormone antagonists are a class of medications that antagonize the gonadotropin-releasing hormone receptor and thus the action of gonadotropin-releasing hormone (GnRH). They are used in the treatment of prostate cancer, endometriosis, uterine fibroids, female infertility in assisted reproduction, and for other indications.
Enzalutamide, sold under the brand name Xtandi, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is indicated for use in conjunction with castration in the treatment of metastatic castration-resistant prostate cancer (mCRPC), nonmetastatic castration-resistant prostate cancer, and metastatic castration-sensitive prostate cancer (mCSPC). It is taken by mouth.
James L. Gulley is an American cancer researcher and the Director of the Medical Oncology Service at National Cancer Institute.
Treatment for prostate cancer may involve active surveillance, surgery, radiation therapy – including brachytherapy and external-beam radiation therapy, proton therapy, high-intensity focused ultrasound (HIFU), cryosurgery, hormonal therapy, chemotherapy, or some combination. Treatments also extend to survivorship based interventions. These interventions are focused on five domains including: physical symptoms, psychological symptoms, surveillance, health promotion and care coordination. However, a published review has found only high levels of evidence for interventions that target physical and psychological symptom management and health promotion, with no reviews of interventions for either care coordination or surveillance. The favored treatment option depends on the stage of the disease, the Gleason score, and the PSA level. Other important factors include the man's age, his general health, and his feelings about potential treatments and their possible side-effects. Because all treatments can have significant side-effects, such as erectile dysfunction and urinary incontinence, treatment discussions often focus on balancing the goals of therapy with the risks of lifestyle alterations.
Simon J. Hall is an American researcher who is the Associate Professor and Kyung Hyun Kim, M.D. Chair of Urology and Assistant Professor, Department of Gene and Cell Medicine at The Mount Sinai School of Medicine, as well as the Director of the Barbara and Maurice Deane Prostate Health and Research Center at The Mount Sinai Medical Center, both in New York City.
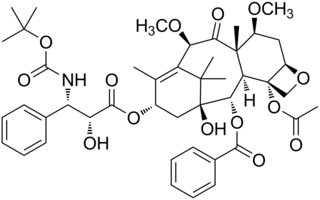
Cabazitaxel, sold under the brand name Jevtana, is a semi-synthetic derivative of a natural taxoid. It is a microtubule inhibitor, and the fourth taxane to be approved as a cancer therapy.
Sipuleucel-T, sold under the brand name Provenge, developed by Dendreon Pharmaceuticals, LLC, is a cell-based cancer immunotherapy for prostate cancer (CaP). It is an autologous cellular immunotherapy.
PROSTVAC is a cancer immunotherapy candidate in clinical development by Bavarian Nordic for the treatment of all prostate cancer although clinical trials are focusing on more advanced cases of metastatic castration-resistant prostate cancer (mCRPC). PROSTVAC is a vaccine designed to enable the immune system to recognize and attack prostate cancer cells by triggering a specific and targeted T cell immune response to cancer cells that express the tumor-associated antigen prostate-specific antigen (PSA).
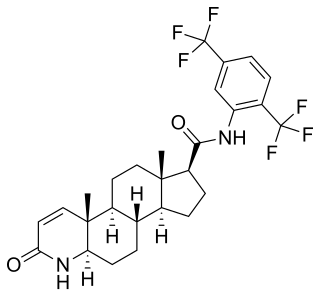
Darolutamide, sold under the brand name Nubeqa, is an antiandrogen medication which is used in the treatment of non-metastatic castration-resistant prostate cancer in men. It is specifically approved to treat non-metastatic castration-resistant prostate cancer (nmCRPC) in conjunction with surgical or medical castration. The medication is taken by mouth twice per day with food.

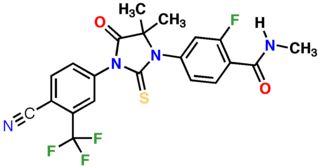
Apalutamide, sold under the brand name Erleada among others, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is specifically indicated for use in conjunction with castration in the treatment of non-metastatic castration-resistant prostate cancer (NM-CRPC). It is taken by mouth.

GTx-758 is a synthetic nonsteroidal estrogen which was under development by GTx, Inc. for the treatment of advanced prostate cancer. As of 2016, it had completed two phase II clinical trials.
The side effects of bicalutamide, a nonsteroidal antiandrogen (NSAA), including its frequent and rare side effects, have been well-studied and characterized. The most common side effects of bicalutamide monotherapy in men include breast tenderness, breast growth, feminization, demasculinization, and hot flashes. Less common side effects of bicalutamide monotherapy in men include sexual dysfunction, depression, fatigue, weakness, and anemia. Bicalutamide is well tolerated and has few side effects in women. General side effects of bicalutamide that may occur in either sex include diarrhea, constipation, abdominal pain, nausea, dry skin, itching, and rash.
Comparison of the nonsteroidal antiandrogen (NSAA) bicalutamide with other antiandrogens reveals differences between the medications in terms of efficacy, tolerability, safety, and other parameters. Relative to the other first-generation NSAAs, flutamide and nilutamide, bicalutamide shows improved potency, efficacy, tolerability, and safety, and has largely replaced these medications in clinical practice. Compared to the second-generation NSAAs, enzalutamide and apalutamide, bicalutamide has inferior potency and efficacy but similar tolerability and safety and a lower propensity for drug interactions.
Oliver Sartor is an American oncologist and research scientist. He is currently the assistant dean for oncology and the C.E. and Bernadine Laborde Professor of Cancer Research, Medicine and Urology Departments at the Tulane School of Medicine in New Orleans, Louisiana. His research has mainly focused on translational science and clinical research trials of advanced prostate cancer since 1990 and he is recognized as an expert in that field through his contributions to the practice and the publishing of over 500 peer-reviewed articles and numerous book chapters and reviews. Sartor also serves as the editor-in-chief of the bimonthly journal Clinical Genitourinary Cancer that mainly focuses on research in genitourinary oncology.

Philip W. Kantoff is a medical oncologist. He is the chairman and chief executive officer (CEO) of Convergent Therapeutics. He served as the Chairman of Medicine at Memorial Sloan Kettering Cancer Center between 2015 and 2021. He is best known for his contributions to the impact of DNA abnormalities in prostate cancer and the discovery of therapies for metastatic hormone-sensitive prostate cancer.
Ronald de Wit is a professor of medical oncology at Erasmus University Medical Center in Rotterdam, Netherlands. He is the founding chairman of the Dutch Uro-Oncology Study Group (DUOS).
References
- 1 2 3 "Mount Sinai Medical Center - Doctor profile".
- ↑ "Castle Connolly Medical Ltd".
- ↑ Taplin ME, Regan MM, Ko YJ, Bubley GJ, Duggan SE, Werner L, Beer TM, Ryan CW, Mathew P, Tu SM, Denmeade SR, Oh WK, Sartor O, Mantzoros CS, Rittmaster R, Kantoff PW, Balk SP (November 2009). "Phase II study of androgen synthesis inhibition with ketoconazole, hydrocortisone, and dutasteride in asymptomatic castration-resistant prostate cancer". Clin. Cancer Res. 15 (22): 7099–105. doi:10.1158/1078-0432.CCR-09-1722. PMC 3644858 . PMID 19887483.
- ↑ "Dana-Farber Cancer Institute Oncologists Present Results of Source MDx's RNA Transcript-Based Six-Gene Test to Predict Survival of Castration-Resistant Prostate Cancer Patients at GU ASCO Sym - News, Search Jobs, Events".
- ↑ Oh WK, Hayes J, Evan C, Manola J, George DJ, Waldron H, Donovan M, Varner J, Orechia J, Katcher B, Lu D, Nevins A, Wright RL, Tormey L, Talcott J, Rubin MA, Loda M, Sellers WR, Richie JP, Kantoff PW, Weeks J (June 2006). "Development of an integrated prostate cancer research information system". Clin Genitourin Cancer. 5 (1): 61–6. doi:10.3816/CGC.2006.n.019. PMID 16859581.
- 1 2 "HealthGrades: Awards & Recognition for Dr. William Oh, MD".