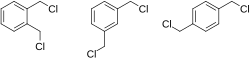
 1,2-, 1,3-, and 1,4-xylylene dichloride | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID | |
| UNII |
|
| UN number | 2928, 2811 |
| |
| |
| Properties | |
| C8H8Cl2 | |
| Molar mass | 175.05 g·mol−1 |
| Density | 1.202 |
| Melting point | 34–37 °C (93–99 °F; 307–310 K) |
| Hazards | |
| GHS labelling: | |
    | |
| Danger | |
| H302, H314, H315, H317, H319, H330, H410 | |
| P260, P261, P264, P270, P271, P272, P273, P280, P284, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P320, P321, P330, P332+P313, P333+P313, P337+P313, P362, P363, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
The chemical compound xylylene dichloride (C6H4(CH2Cl)2) is a white to light yellow sandlike solid. [1] This compound can be classified as a benzyl halide. [2] [3] Xylylene dichloride is used as a vulcanizing agent to harden rubbers. It catalyzes the crosslinking of phenolic resins. [2]