Related Research Articles
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. This group of enzymes usually utilizes NADP+ or NAD+ as cofactors. Transmembrane oxidoreductases create electron transport chains in bacteria, chloroplasts and mitochondria, including respiratory complexes I, II and III. Some others can associate with biological membranes as peripheral membrane proteins or be anchored to the membranes through a single transmembrane helix.

Nitric oxide synthases (NOSs) are a family of enzymes catalyzing the production of nitric oxide (NO) from L-arginine. NO is an important cellular signaling molecule. It helps modulate vascular tone, insulin secretion, airway tone, and peristalsis, and is involved in angiogenesis and neural development. It may function as a retrograde neurotransmitter. Nitric oxide is mediated in mammals by the calcium-calmodulin controlled isoenzymes eNOS and nNOS. The inducible isoform, iNOS, involved in immune response, binds calmodulin at physiologically relevant concentrations, and produces NO as an immune defense mechanism, as NO is a free radical with an unpaired electron. It is the proximate cause of septic shock and may function in autoimmune disease.

Nicotinamide adenine dinucleotide phosphate, abbreviated NADP+ or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). NADPH is the reduced form, whereas NADP+ is the oxidized form. NADP+ is used by all forms of cellular life.
Ferredoxins are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied to the "iron protein" first purified in 1962 by Mortenson, Valentine, and Carnahan from the anaerobic bacterium Clostridium pasteurianum.

Biliverdin reductase (BVR) is an enzyme found in all tissues under normal conditions, but especially in reticulo-macrophages of the liver and spleen. BVR facilitates the conversion of biliverdin to bilirubin via the reduction of a double-bond between the second and third pyrrole ring into a single-bond.

Vitamin K epoxide reductase (VKOR) is an enzyme that reduces vitamin K after it has been oxidised in the carboxylation of glutamic acid residues in blood coagulation enzymes. VKOR is a member of a large family of predicted enzymes that are present in vertebrates, Drosophila, plants, bacteria and archaea. In some plant and bacterial homologues, the VKOR domain is fused with domains of the thioredoxin family of oxidoreductases.

Cytochrome P450 reductase is a membrane-bound enzyme required for electron transfer from NADPH to cytochrome P450 and other heme proteins including heme oxygenase in the endoplasmic reticulum of the eukaryotic cell.
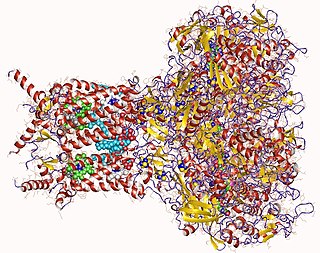
Formate dehydrogenases are a set of enzymes that catalyse the oxidation of formate to carbon dioxide, donating the electrons to a second substrate, such as NAD+ in formate:NAD+ oxidoreductase (EC 1.17.1.9) or to a cytochrome in formate:ferricytochrome-b1 oxidoreductase (EC 1.2.2.1). This family of enzymes has attracted attention as inspiration or guidance on methods for the carbon dioxide fixation, relevant to global warming.
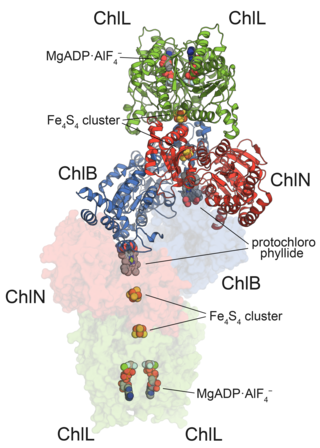
In enzymology, protochlorophyllide reductases (POR) are enzymes that catalyze the conversion from protochlorophyllide to chlorophyllide a. They are oxidoreductases participating in the biosynthetic pathway to chlorophylls.
In enzymology, a ferredoxin-NADP+ reductase (EC 1.18.1.2) abbreviated FNR, is an enzyme that catalyzes the chemical reaction
In enzymology, a ferric-chelate reductase (EC 1.16.1.7) is an enzyme that catalyzes the chemical reaction
Flavin reductase a class of enzymes. There are a variety of flavin reductases, which bind free flavins and through hydrogen bonding, catalyze the reduction of these molecules to a reduced flavin. Riboflavin, or vitamin B, and flavin mononucleotide are two of the most well known flavins in the body and are used in a variety of processes which include metabolism of fat and ketones and the reduction of methemoglobin in erythrocytes. Flavin reductases are similar and often confused for ferric reductases because of their similar catalytic mechanism and structures.

Thiosulfate dehydrogenase is an enzyme that catalyzes the chemical reaction:

Dual oxidase 1, also known as DUOX1 or ThOX1, is an enzyme which in humans is encoded by the DUOX1 gene. DUOX1 was first identified in the mammalian thyroid gland. In humans, two isoforms are found; hDUOX1 and hDUOX2. Human DUOX protein localization is not exclusive to thyroid tissue; hDUOX1 is prominent in airway epithelial cells and hDUOX2 in the salivary glands and gastrointestinal tract.

Metalloreductase STEAP3 is an enzyme that in humans is encoded by the STEAP3 gene.

Metalloreductase STEAP2 is an enzyme that in humans is encoded by the STEAP2 gene.

Fumarate reductase (quinol) (EC 1.3.5.4, QFR,FRD, menaquinol-fumarate oxidoreductase, quinol:fumarate reductase) is an enzyme with systematic name succinate:quinone oxidoreductase. This enzyme catalyzes the following chemical reaction:
Adrenodoxin-NADP+ reductase (EC 1.18.1.6, adrenodoxin reductase, nicotinamide adenine dinucleotide phosphate-adrenodoxin reductase, ADR, NADPH:adrenal ferredoxin oxidoreductase) is an enzyme with systematic name adrendoxin:NADP+ oxidoreductase. This enzyme catalyses the following chemical reaction

Biliverdin reductase B is a protein that in humans is encoded by the BLVRB gene.
The Disulfide bond oxidoreductase D (DsbD) family is a member of the Lysine Exporter (LysE) Superfamily. A representative list of proteins belonging to the DsbD family can be found in the Transporter Classification Base.
References
- ↑ Drew, David; Sjöstrand, Dan; Nilsson, Johan; Urbig, Thomas; Chin, Chen-ni; de Gier, Jan-Willem; von Heijne, Gunnar (2002-03-05). "Rapid topology mapping of Escherichia coli inner-membrane proteins by prediction and PhoA/GFP fusion analysis". Proceedings of the National Academy of Sciences of the United States of America. 99 (5): 2690–2695. Bibcode:2002PNAS...99.2690D. doi: 10.1073/pnas.052018199 . ISSN 0027-8424. PMC 122409 . PMID 11867724.
- 1 2 3 4 von Rozycki, Torsten; Yen, Ming-Ren; Lende, Erik E.; Saier, Milton H. (2004-01-01). "The YedZ family: possible heme binding proteins that can be fused to transporters and electron carriers". Journal of Molecular Microbiology and Biotechnology. 8 (3): 129–140. doi:10.1159/000085786. ISSN 1464-1801. PMID 16088215. S2CID 34128668.
- ↑ Hubert, R. S.; Vivanco, I.; Chen, E.; Rastegar, S.; Leong, K.; Mitchell, S. C.; Madraswala, R.; Zhou, Y.; Kuo, J. (1999-12-07). "STEAP: a prostate-specific cell-surface antigen highly expressed in human prostate tumors". Proceedings of the National Academy of Sciences of the United States of America. 96 (25): 14523–14528. Bibcode:1999PNAS...9614523H. doi: 10.1073/pnas.96.25.14523 . ISSN 0027-8424. PMC 24469 . PMID 10588738.
- ↑ Yang, D.; Holt, G. E.; Velders, M. P.; Kwon, E. D.; Kast, W. M. (2001-08-01). "Murine six-transmembrane epithelial antigen of the prostate, prostate stem cell antigen, and prostate-specific membrane antigen: prostate-specific cell-surface antigens highly expressed in prostate cancer of transgenic adenocarcinoma mouse prostate mice". Cancer Research. 61 (15): 5857–5860. ISSN 0008-5472. PMID 11479226.
- ↑ Zhang, Xuezhi; Krause, Karl-Heinz; Xenarios, Ioannis; Soldati, Thierry; Boeckmann, Brigitte (2013-01-01). "Evolution of the ferric reductase domain (FRD) superfamily: modularity, functional diversification, and signature motifs". PLOS ONE. 8 (3): e58126. Bibcode:2013PLoSO...858126Z. doi: 10.1371/journal.pone.0058126 . ISSN 1932-6203. PMC 3591440 . PMID 23505460.
- 1 2 Kleven, Mark D.; Dlakić, Mensur; Lawrence, C. Martin (2015-09-11). "Characterization of a single b-type heme, FAD, and metal binding sites in the transmembrane domain of six-transmembrane epithelial antigen of the prostate (STEAP) family proteins". The Journal of Biological Chemistry. 290 (37): 22558–22569. doi: 10.1074/jbc.M115.664565 . ISSN 1083-351X. PMC 4566230 . PMID 26205815.
As of this edit, this article uses content from "5.B.7. The YedZ (YedZ) Family" , which is licensed in a way that permits reuse under the Creative Commons Attribution-ShareAlike 3.0 Unported License, but not under the GFDL. All relevant terms must be followed.