The haloalkanes are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.

In chemistry, iron(II) refers to the element iron in its +2 oxidation state. The adjective ferrous or the prefix ferro- is often used to specify such compounds, as in ferrous chloride for iron(II) chloride (FeCl2). The adjective ferric is used instead for iron(III) salts, containing the cation Fe3+. The word ferrous is derived from the Latin word ferrum, meaning "iron".

Ytterbium(III) oxide is the chemical compound with the formula Yb2O3. It is one of the more commonly encountered compounds of ytterbium. It occurs naturally in trace amounts in the mineral gadolinite. It was first isolated from this in 1878 by Jean Charles Galissard de Marignac.
The Corey–House synthesis (also called the Corey–Posner–Whitesides–House reaction and other permutations) is an organic reaction that involves the reaction of a lithium diorganylcuprate () with an organic halide or pseudohalide () to form a new alkane, as well as an ill-defined organocopper species and lithium (pseudo)halide as byproducts.

Lithium bromide (LiBr) is a chemical compound of lithium and bromine. Its extreme hygroscopic character makes LiBr useful as a desiccant in certain air conditioning systems.
Cyanogen bromide is the inorganic compound with the formula (CN)Br or BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides, and synthesize other compounds. The compound is classified as a pseudohalogen.

Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound, with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry.

Barium iodide is an inorganic compound with the formula BaI2. The compound exists as an anhydrous and a hydrate (BaI2(H2O)2), both of which are white solids. When heated, hydrated barium iodide converts to the anhydrous salt. The hydrated form is freely soluble in water, ethanol, and acetone.
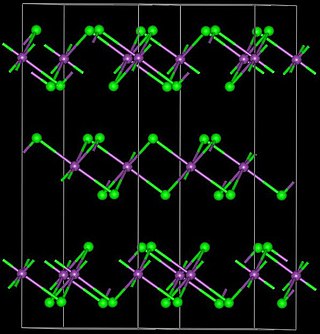
Ytterbium(III) bromide (YbBr3) is an inorganic chemical compound.

Organocopper chemistry is the study of the physical properties, reactions, and synthesis of organocopper compounds, which are organometallic compounds containing a carbon to copper chemical bond. They are reagents in organic chemistry.
A cyanogen halide is a molecule consisting of cyanide and a halogen. Cyanogen halides are chemically classified as pseudohalogens.
Reactions of organocopper reagents involve species containing copper-carbon bonds acting as nucleophiles in the presence of organic electrophiles. Organocopper reagents are now commonly used in organic synthesis as mild, selective nucleophiles for substitution and conjugate addition reactions.

Lithium cyanide is an inorganic compound with the chemical formula LiCN. It is a toxic, white coloured, hygroscopic, water-soluble salt that finds only niche uses.

Europium(II) chloride is an inorganic compound with a chemical formula EuCl2. When it is irradiated by ultraviolet light, it has bright blue fluorescence.
Dysprosium(II) chloride (DyCl2), also known as dysprosium dichloride, is an ionic chemical compound of dysprosium and chlorine. This salt is a reduced compound, as the normal oxidation state of dysprosium in dysprosium compounds is +3.
Ytterbium(III) phosphide is an inorganic compound of ytterbium and phosphorus with the chemical formula YbP. This is one of the phosphides of ytterbium.
Ytterbium compounds are chemical compounds that contain the element ytterbium (Yb). The chemical behavior of ytterbium is similar to that of the rest of the lanthanides. Most ytterbium compounds are found in the +3 oxidation state, and its salts in this oxidation state are nearly colorless. Like europium, samarium, and thulium, the trihalides of ytterbium can be reduced to the dihalides by hydrogen, zinc dust, or by the addition of metallic ytterbium. The +2 oxidation state occurs only in solid compounds and reacts in some ways similarly to the alkaline earth metal compounds; for example, ytterbium(II) oxide (YbO) shows the same structure as calcium oxide (CaO).
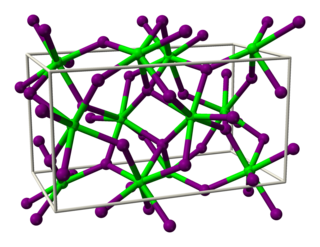
Thulium dibromide is an inorganic compound, with the chemical formula of TmBr2. It is a dark green solid that is easy to dissolve, with the SrI2 structure and it needs to be stored in an inert atmosphere.

Ytterbium(II) bromide is an inorganic compound with the chemical formula YbBr2.










