Related Research Articles
A quality management system (QMS) is a collection of business processes focused on consistently meeting customer requirements and enhancing their satisfaction. It is aligned with an organization's purpose and strategic direction. It is expressed as the organizational goals and aspirations, policies, processes, documented information, and resources needed to implement and maintain it. Early quality management systems emphasized predictable outcomes of an industrial product production line, using simple statistics and random sampling. By the 20th century, labor inputs were typically the most costly inputs in most industrialized societies, so focus shifted to team cooperation and dynamics, especially the early signaling of problems via a continual improvement cycle. In the 21st century, QMS has tended to converge with sustainability and transparency initiatives, as both investor and customer satisfaction and perceived quality are increasingly tied to these factors. Of QMS regimes, the ISO 9000 family of standards is probably the most widely implemented worldwide – the ISO 19011 audit regime applies to both and deals with quality and sustainability and their integration.
Software testing is the act of examining the artifacts and the behavior of the software under test by validation and verification. Software testing can also provide an objective, independent view of the software to allow the business to appreciate and understand the risks of software implementation. Test techniques include, but are not necessarily limited to:

Quality control (QC) is a process by which entities review the quality of all factors involved in production. ISO 9000 defines quality control as "a part of quality management focused on fulfilling quality requirements".

Nondestructive testing (NDT) is any of a wide group of analysis techniques used in science and technology industry to evaluate the properties of a material, component or system without causing damage. The terms nondestructive examination (NDE), nondestructive inspection (NDI), and nondestructive evaluation (NDE) are also commonly used to describe this technology. Because NDT does not permanently alter the article being inspected, it is a highly valuable technique that can save both money and time in product evaluation, troubleshooting, and research. The six most frequently used NDT methods are eddy-current, magnetic-particle, liquid penetrant, radiographic, ultrasonic, and visual testing. NDT is commonly used in forensic engineering, mechanical engineering, petroleum engineering, electrical engineering, civil engineering, systems engineering, aeronautical engineering, medicine, and art. Innovations in the field of nondestructive testing have had a profound impact on medical imaging, including on echocardiography, medical ultrasonography, and digital radiography.
Quality assurance (QA) is the term used in both manufacturing and service industries to describe the systematic efforts taken to assure that the product(s) delivered to customer(s) meet with the contractual and other agreed upon performance, design, reliability, and maintainability expectations of that customer. The core purpose of Quality Assurance is to prevent mistakes and defects in the development and production of both manufactured products, such as automobiles and shoes, and delivered services, such as automotive repair and athletic shoe design. Assuring quality and therefore avoiding problems and delays when delivering products or services to customers is what ISO 9000 defines as that "part of quality management focused on providing confidence that quality requirements will be fulfilled". This defect prevention aspect of quality assurance differs from the defect detection aspect of quality control and has been referred to as a shift left since it focuses on quality efforts earlier in product development and production and on avoiding defects in the first place rather than correcting them after the fact.

Hazard analysis and critical control points, or HACCP, is a systematic preventive approach to food safety from biological, chemical, and physical hazards in production processes that can cause the finished product to be unsafe and designs measures to reduce these risks to a safe level. In this manner, HACCP attempts to avoid hazards rather than attempting to inspect finished products for the effects of those hazards. The HACCP system can be used at all stages of a food chain, from food production and preparation processes including packaging, distribution, etc. The Food and Drug Administration (FDA) and the United States Department of Agriculture (USDA) require mandatory HACCP programs for juice and meat as an effective approach to food safety and protecting public health. Meat HACCP systems are regulated by the USDA, while seafood and juice are regulated by the FDA. All other food companies in the United States that are required to register with the FDA under the Public Health Security and Bioterrorism Preparedness and Response Act of 2002, as well as firms outside the US that export food to the US, are transitioning to mandatory hazard analysis and risk-based preventive controls (HARPC) plans.

An inspection is, most generally, an organized examination or formal evaluation exercise. In engineering activities inspection involves the measurements, tests, and gauges applied to certain characteristics in regard to an object or activity. The results are usually compared to specified requirements and standards for determining whether the item or activity is in line with these targets, often with a Standard Inspection Procedure in place to ensure consistent checking. Inspections are usually non-destructive.

Engineering tolerance is the permissible limit or limits of variation in:
- a physical dimension;
- a measured value or physical property of a material, manufactured object, system, or service;
- other measured values ;
- in engineering and safety, a physical distance or space (tolerance), as in a truck (lorry), train or boat under a bridge as well as a train in a tunnel ;
- in mechanical engineering, the space between a bolt and a nut or a hole, etc.
Statistical process control (SPC) or statistical quality control (SQC) is the application of statistical methods to monitor and control the quality of a production process. This helps to ensure that the process operates efficiently, producing more specification-conforming products with less waste scrap. SPC can be applied to any process where the "conforming product" output can be measured. Key tools used in SPC include run charts, control charts, a focus on continuous improvement, and the design of experiments. An example of a process where SPC is applied is manufacturing lines.
Reliability engineering is a sub-discipline of systems engineering that emphasizes the ability of equipment to function without failure. Reliability describes the ability of a system or component to function under stated conditions for a specified period of time. Reliability is closely related to availability, which is typically described as the ability of a component or system to function at a specified moment or interval of time.

MIL-STD-105 was a United States defense standard that provided procedures and tables for sampling by attributes based on Walter A. Shewhart, Harry Romig, and Harold F. Dodge sampling inspection theories and mathematical formulas. Widely adopted outside of military procurement applications.
Environmental stress screening (ESS) refers to the process of exposing a newly manufactured or repaired product or component to stresses such as thermal cycling and vibration in order to force latent defects to manifest themselves by permanent or catastrophic failure during the screening process. The surviving population, upon completion of screening, can be assumed to have a higher reliability than a similar unscreened population.
Certified Quality Engineer, often abbreviated CQE, is a certification given by the American Society for Quality (ASQ). These engineers are professionally educated in quality engineering and quality control.
The combination of quality control and genetic algorithms led to novel solutions of complex quality control design and optimization problems. Quality is the degree to which a set of inherent characteristics of an entity fulfils a need or expectation that is stated, general implied or obligatory. ISO 9000 defines quality control as "A part of quality management focused on fulfilling quality requirements". Genetic algorithms are search algorithms, based on the mechanics of natural selection and natural genetics.

Dorian Shainin was an American quality consultant, aeronautics engineer, author, and college professor most notable for his contributions in the fields of industrial problem solving, product reliability, and quality engineering, particularly the creation and development of the "Red X" concept.
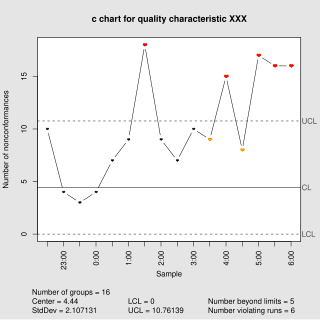
In statistical quality control, the c-chart is a type of control chart used to monitor "count"-type data, typically total number of nonconformities per unit. It is also occasionally used to monitor the total number of events occurring in a given unit of time.
Pre-shipment inspection is a part of supply chain management and an important quality control method for checking the quality of goods clients buy from suppliers.
Acceptance sampling uses statistical sampling to determine whether to accept or reject a production lot of material. It has been a common quality control technique used in industry.
Analytical quality control, commonly shortened to AQC, refers to all those processes and procedures designed to ensure that the results of laboratory analysis are consistent, comparable, accurate and within specified limits of precision. Constituents submitted to the analytical laboratory must be accurately described to avoid faulty interpretations, approximations, or incorrect results. The qualitative and quantitative data generated from the laboratory can then be used for decision making. In the chemical sense, quantitative analysis refers to the measurement of the amount or concentration of an element or chemical compound in a matrix that differs from the element or compound. Fields such as industry, medicine, and law enforcement can make use of AQC.
In statistics, a variables sampling plan is an acceptance sampling technique. Plans for variables are intended for quality characteristics that are measured on a continuous scale. This plan requires the knowledge of the statistical model. The historical evolution of this technique dates back to the seminal work of W. Allen Wallis (1943). The purpose of a plan for variables is to assess whether the process is operating far enough from the specification limit. Plans for variables may produce a similar OC curve to attribute plans with significantly less sample size.
References
- 1 2 Dodge, Y. (2003) The Oxford Dictionary of Statistical Terms. OUP. ISBN 0-19-920613-9
- ↑ Chandrupatla, T.R. (2009) Quality and Reliability in Engineering, CUP. ISBN 978-0-521-51522-1
- ↑ ANSI/ASQ Z1.4-2008 Sampling Procedures and Tables for Inspection by Attributes.
- ↑ http://www.aqlinspectorsrule.com/Z1-4-2008.html Archived 2009-06-01 at the Wayback Machine AQL Inspectors Rule