
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics. The word crystallography is derived from the Ancient Greek word κρύσταλλος, with its meaning extending to all solids with some degree of transparency, and γράφειν. In July 2012, the United Nations recognised the importance of the science of crystallography by proclaiming that 2014 would be the International Year of Crystallography.

Structural biology is a field that is many centuries old which, as defined by the Journal of Structural Biology, deals with structural analysis of living material at every level of organization. Early structural biologists throughout the 19th and early 20th centuries were primarily only able to study structures to the limit of the naked eye's visual acuity and through magnifying glasses and light microscopes.
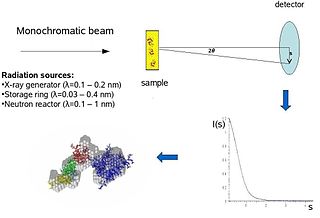
Biological small-angle scattering is a small-angle scattering method for structure analysis of biological materials. Small-angle scattering is used to study the structure of a variety of objects such as solutions of biological macromolecules, nanocomposites, alloys, and synthetic polymers. Small-angle X-ray scattering (SAXS) and small-angle neutron scattering (SANS) are the two complementary techniques known jointly as small-angle scattering (SAS). SAS is an analogous method to X-ray and neutron diffraction, wide angle X-ray scattering, as well as to static light scattering. In contrast to other X-ray and neutron scattering methods, SAS yields information on the sizes and shapes of both crystalline and non-crystalline particles. When used to study biological materials, which are very often in aqueous solution, the scattering pattern is orientation averaged.

Transmission electron cryomicroscopy (CryoTEM), commonly known as cryo-EM, is a form of cryogenic electron microscopy, more specifically a type of transmission electron microscopy (TEM) where the sample is studied at cryogenic temperatures. Cryo-EM is gaining popularity in structural biology.
The National Academy of Sciences Award in Chemical Sciences is awarded for innovative research in the chemical sciences that in the broadest sense contributes to a better understanding of the natural sciences and to the benefit of humanity.

The Max Planck Institute of Biophysics is located in Frankfurt, Germany. It was founded as the Kaiser Wilhelm Institute of Biophysics in 1937, and moved into a new building in 2003. It is an institute of the Max Planck Society.

Richard Henderson is a British molecular biologist and biophysicist and pioneer in the field of electron microscopy of biological molecules. Henderson shared the Nobel Prize in Chemistry in 2017 with Jacques Dubochet and Joachim Frank.

Eva Nogales is a Spanish-American biophysicist at the Lawrence Berkeley National Laboratory and a professor at the University of California, Berkeley, where she served as head of the Division of Biochemistry, Biophysics and Structural Biology of the Department of Molecular and Cell Biology (2015–2020). She is a Howard Hughes Medical Institute investigator.
Xuong Nguyen-Huu is a pioneer of protein crystallography technology. His research focuses on the development of novel methods, such as protein crystallography and cryo-electron microscopy, for the determination of protein structures and biological macromolecules.
The NAS Award in Molecular Biology is awarded by the U.S. National Academy of Sciences "for recent notable discovery in molecular biology by a young scientist who is a citizen of the United States." It has been awarded annually since its inception in 1962.
The following outline is provided as an overview of and topical guide to biophysics:

The term macromolecular assembly (MA) refers to massive chemical structures such as viruses and non-biologic nanoparticles, cellular organelles and membranes and ribosomes, etc. that are complex mixtures of polypeptide, polynucleotide, polysaccharide or other polymeric macromolecules. They are generally of more than one of these types, and the mixtures are defined spatially, and with regard to their underlying chemical composition and structure. Macromolecules are found in living and nonliving things, and are composed of many hundreds or thousands of atoms held together by covalent bonds; they are often characterized by repeating units. Assemblies of these can likewise be biologic or non-biologic, though the MA term is more commonly applied in biology, and the term supramolecular assembly is more often applied in non-biologic contexts. MAs of macromolecules are held in their defined forms by non-covalent intermolecular interactions, and can be in either non-repeating structures, or in repeating linear, circular, spiral, or other patterns. The process by which MAs are formed has been termed molecular self-assembly, a term especially applied in non-biologic contexts. A wide variety of physical/biophysical, chemical/biochemical, and computational methods exist for the study of MA; given the scale of MAs, efforts to elaborate their composition and structure and discern mechanisms underlying their functions are at the forefront of modern structure science.
Cell biophysics is a sub-field of biophysics that focuses on physical principles underlying cell function. Sub-areas of current interest include statistical models of intracellular signaling dynamics, intracellular transport, cell mechanics, molecular motors, biological electricity and genetic network theory. The field has benefited greatly from recent advances in live-cell molecular imaging techniques that allow spatial and temporal measurement of macromolecules and macromolecular function. Specialized imaging methods like FRET, FRAP, photoactivation and single molecule imaging have proven useful for mapping macromolecular transport, dynamic conformational changes in proteins and macromolecular interactions. Super-resolution microscopy allows imaging of cell structures below the optical resolution of light. Combining novel experimental tools with mathematical models grounded in the physical sciences has enabled significant recent breakthroughs in the field. Multiple centers across the world are advancing the research area

G. Marius Clore MAE, FRSC, FRS is a British-born, Anglo-American molecular biophysicist and structural biologist. He was born in London, U.K. and is a dual U.S./U.K. Citizen. He is a Member of the National Academy of Sciences, a Fellow of the Royal Society, a NIH Distinguished Investigator, and the Chief of the Molecular and Structural Biophysics Section in the Laboratory of Chemical Physics of the National Institute of Diabetes and Digestive and Kidney Diseases at the U.S. National Institutes of Health. He is known for his foundational work in three-dimensional protein and nucleic acid structure determination by biomolecular NMR spectroscopy, for advancing experimental approaches to the study of large macromolecules and their complexes by NMR, and for developing NMR-based methods to study rare conformational states in protein-nucleic acid and protein-protein recognition. Clore's discovery of previously undetectable, functionally significant, rare transient states of macromolecules has yielded fundamental new insights into the mechanisms of important biological processes, and in particular the significance of weak interactions and the mechanisms whereby the opposing constraints of speed and specificity are optimized. Further, Clore's work opens up a new era of pharmacology and drug design as it is now possible to target structures and conformations that have been heretofore unseen.

Joachim Frank ; born September 12, 1940) is a German-American biophysicist at Columbia University and a Nobel laureate. He is regarded as the founder of single-particle cryo-electron microscopy (cryo-EM), for which he shared the Nobel Prize in Chemistry in 2017 with Jacques Dubochet and Richard Henderson. He also made significant contributions to structure and function of the ribosome from bacteria and eukaryotes.

Cryogenic electron microscopy (cryo-EM) is a cryomicroscopy technique applied on samples cooled to cryogenic temperatures. For biological specimens, the structure is preserved by embedding in an environment of vitreous ice. An aqueous sample solution is applied to a grid-mesh and plunge-frozen in liquid ethane or a mixture of liquid ethane and propane. While development of the technique began in the 1970s, recent advances in detector technology and software algorithms have allowed for the determination of biomolecular structures at near-atomic resolution. This has attracted wide attention to the approach as an alternative to X-ray crystallography or NMR spectroscopy for macromolecular structure determination without the need for crystallization.

Tamir Gonen is an American structural biochemist and membrane biophysicist best known for his contributions to structural biology of membrane proteins, membrane biochemistry and electron cryo-microscopy (cryoEM) particularly in electron crystallography of 2D crystals and for the development of 3D electron crystallography from microscopic crystals known as MicroED. Gonen is an Investigator of the Howard Hughes Medical Institute, a professor at the University of California, Los Angeles, the founding director of the MicroED Imaging Center at UCLA and a Member of the Royal Society of New Zealand.
Barry H. Honig is an American biochemist, molecular biophysicist, and computational biophysicist, who develops theoretical methods and computer software for "analyzing the structure and function of biological macromolecules."
Wolfgang P. Baumeister is a German molecular biologist and biophysicist. His research has been pivotal in the development of Cryoelectron tomography.











