The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive elements polonium (Po) and livermorium (Lv). Often, oxygen is treated separately from the other chalcogens, sometimes even excluded from the scope of the term "chalcogen" altogether, due to its very different chemical behavior from sulfur, selenium, tellurium, and polonium. The word "chalcogen" is derived from a combination of the Greek word khalkόs (χαλκός) principally meaning copper, and the Latinized Greek word genēs, meaning born or produced.
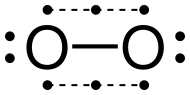
Oxygen is a chemical element; it has symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is the most abundant element in Earth's crust, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula O
2. Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.
Hund's rule of maximum multiplicity is a rule based on observation of atomic spectra, which is used to predict the ground state of an atom or molecule with one or more open electronic shells. The rule states that for a given electron configuration, the lowest energy term is the one with the greatest value of spin multiplicity. This implies that if two or more orbitals of equal energy are available, electrons will occupy them singly before filling them in pairs. The rule, discovered by Friedrich Hund in 1925, is of important use in atomic chemistry, spectroscopy, and quantum chemistry, and is often abbreviated to Hund's rule, ignoring Hund's other two rules.
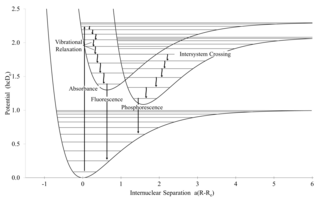
Intersystem crossing (ISC) is an isoenergetic radiationless process involving a transition between the two electronic states with different spin multiplicity.

The ozone–oxygen cycle is the process by which ozone is continually regenerated in Earth's stratosphere, converting ultraviolet radiation (UV) into heat. In 1930 Sydney Chapman resolved the chemistry involved. The process is commonly called the Chapman cycle by atmospheric scientists.

Sulfur monoxide is an inorganic compound with formula SO. It is only found as a dilute gas phase. When concentrated or condensed, it converts to S2O2 (disulfur dioxide). It has been detected in space but is rarely encountered intact otherwise.

Oxygen fluorides are compounds of elements oxygen and fluorine with the general formula OnF2, where n = 1 to 6. Many different oxygen fluorides are known:

Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemical with the formula O=O (also written as 1
[O
2] or 1
O
2), which is in a quantum state where all electrons are spin paired. It is kinetically unstable at ambient temperature, but the rate of decay is slow.
The tetraoxygen molecule (O4), also called oxozone, is an allotrope of oxygen consisting of four oxygen atoms.

Triplet oxygen, 3O2, refers to the S = 1 electronic ground state of molecular oxygen (dioxygen). Molecules of triplet oxygen contain two unpaired electrons, making triplet oxygen an unusual example of a stable and commonly encountered diradical: it is more stable as a triplet than a singlet. According to molecular orbital theory, the electron configuration of triplet oxygen has two electrons occupying two π molecular orbitals (MOs) of equal energy (that is, degenerate MOs). In accordance with Hund's rules, they remain unpaired and spin-parallel, which accounts for the paramagnetism of molecular oxygen. These half-filled orbitals are antibonding in character, reducing the overall bond order of the molecule to 2 from the maximum value of 3 that would occur when these antibonding orbitals remain fully unoccupied, as in dinitrogen. The molecular term symbol for triplet oxygen is 3Σ−
g.
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule.
In chemistry, homonuclear molecules, or homonuclear species, are molecules composed of only one element. Homonuclear molecules may consist of various numbers of atoms. The size of the molecule an element can form depends on the element's properties, and some elements form molecules of more than one size. The most familiar homonuclear molecules are diatomic molecule, which consist of two atoms, although not all diatomic molecules are homonuclear. Homonuclear diatomic molecules include hydrogen, oxygen, nitrogen and all of the halogens. Ozone is a common triatomic homonuclear molecule. Homonuclear tetratomic molecules include arsenic and phosphorus.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of molecular orbitals, although the electrons involved may be redistributed among the orbitals. This tool is very well suited for simple diatomic molecules such as dihydrogen, dioxygen, and carbon monoxide but becomes more complex when discussing even comparatively simple polyatomic molecules, such as methane. MO diagrams can explain why some molecules exist and others do not. They can also predict bond strength, as well as the electronic transitions that can take place.
Oxygenevolution is the process of generating molecular oxygen (O2) by a chemical reaction, usually from water. Oxygen evolution from water is effected by oxygenic photosynthesis, electrolysis of water, and thermal decomposition of various oxides. The biological process supports aerobic life. When relatively pure oxygen is required industrially, it is isolated by distilling liquefied air.
In spectroscopy and quantum chemistry, the multiplicity of an energy level is defined as 2S+1, where S is the total spin angular momentum. States with multiplicity 1, 2, 3, 4, 5 are respectively called singlets, doublets, triplets, quartets and quintets.
Dioxygen plays an important role in the energy metabolism of living organisms. Free oxygen is produced in the biosphere through photolysis of water during photosynthesis in cyanobacteria, green algae, and plants. During oxidative phosphorylation in cellular respiration, oxygen is reduced to water, thus closing the biological water-oxygen redox cycle.
An electric effect influences the structure, reactivity, or properties of a molecule but is neither a traditional bond nor a steric effect. In organic chemistry, the term stereoelectronic effect is also used to emphasize the relation between the electronic structure and the geometry (stereochemistry) of a molecule.

Disulfur is the diatomic molecule with the formula S2. It is analogous to the dioxygen molecule but rarely occurs at room temperature. This violet gas is the dominant species in hot sulfur vapors. S2 is one of the minor components of the atmosphere of Io, which is predominantly composed of SO2. The instability of S2 is usually described in the context of the double bond rule.

In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes.
Dioxygen complexes are coordination compounds that contain O2 as a ligand. The study of these compounds is inspired by oxygen-carrying proteins such as myoglobin, hemoglobin, hemerythrin, and hemocyanin. Several transition metals form complexes with O2, and many of these complexes form reversibly. The binding of O2 is the first step in many important phenomena, such as cellular respiration, corrosion, and industrial chemistry. The first synthetic oxygen complex was demonstrated in 1938 with cobalt(II) complex reversibly bound O2.