Adenosine triphosphate (ATP) is a nucleotide that provides energy to drive and support many processes in living cells, such as muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all known forms of life, it is often referred to as the "molecular unit of currency" of intracellular energy transfer.

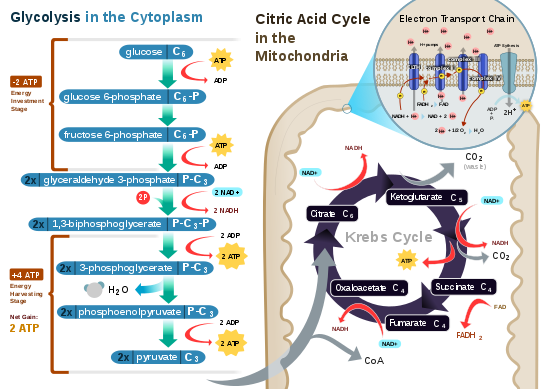
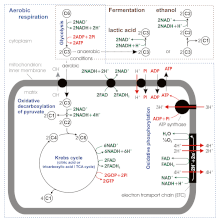
The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of biochemical reactions to release the energy stored in nutrients through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. The chemical energy released is available under the form of ATP. The Krebs cycle is used by organisms that respire (as opposed to organisms that ferment) to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism. Even though it is branded as a "cycle", it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized.

Glycolysis is the metabolic pathway that converts glucose into pyruvate and, in most organisms, occurs in the liquid part of cells. The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes.

Adenosine diphosphate (ADP), also known as adenosine pyrophosphate (APP), is an important organic compound in metabolism and is essential to the flow of energy in living cells. ADP consists of three important structural components: a sugar backbone attached to adenine and two phosphate groups bonded to the 5 carbon atom of ribose. The diphosphate group of ADP is attached to the 5’ carbon of the sugar backbone, while the adenine attaches to the 1’ carbon.
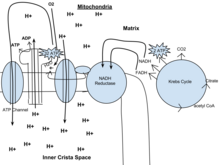
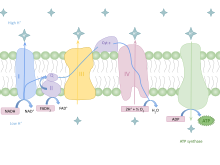
An electron transport chain (ETC) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. Many of the enzymes in the electron transport chain are embedded within the membrane.

An aerobic organism or aerobe is an organism that can survive and grow in an oxygenated environment. The ability to exhibit aerobic respiration may yield benefits to the aerobic organism, as aerobic respiration yields more energy than anaerobic respiration. Energy production of the cell involves the synthesis of ATP by an enzyme called ATP synthase. In aerobic respiration, ATP synthase is coupled with an electron transport chain in which oxygen acts as a terminal electron acceptor. In July 2020, marine biologists reported that aerobic microorganisms (mainly), in "quasi-suspended animation", were found in organically poor sediments, up to 101.5 million years old, 250 feet below the seafloor in the South Pacific Gyre (SPG), and could be the longest-living life forms ever found.
Anaerobic glycolysis is the transformation of glucose to lactate when limited amounts of oxygen (O2) are available. Anaerobic glycolysis is an effective means of energy production only during short, intense exercise, providing energy for a period ranging from 10 seconds to 2 minutes. This is much faster than aerobic metabolism. The anaerobic glycolysis (lactic acid) system is dominant from about 10–30 seconds during a maximal effort. It replenishes very quickly over this period and produces 2 ATP molecules per glucose molecule, or about 5% of glucose's energy potential (38 ATP molecules). The speed at which ATP is produced is about 100 times that of oxidative phosphorylation.

A crista is a fold in the inner membrane of a mitochondrion. The name is from the Latin for crest or plume, and it gives the inner membrane its characteristic wrinkled shape, providing a large amount of surface area for chemical reactions to occur on. This aids aerobic cellular respiration, because the mitochondrion requires oxygen. Cristae are studded with proteins, including ATP synthase and a variety of cytochromes.
Digestion is the breakdown of carbohydrates to yield an energy-rich compound called ATP. The production of ATP is achieved through the oxidation of glucose molecules. In oxidation, the electrons are stripped from a glucose molecule to reduce NAD+ and FAD. NAD+ and FAD possess a high energy potential to drive the production of ATP in the electron transport chain. ATP production occurs in the mitochondria of the cell. There are two methods of producing ATP: aerobic and anaerobic. In aerobic respiration, oxygen is required. Using oxygen increases ATP production from 4 ATP molecules to about 30 ATP molecules. In anaerobic respiration, oxygen is not required. When oxygen is absent, the generation of ATP continues through fermentation. There are two types of fermentation: alcohol fermentation and lactic acid fermentation.
Carbohydrate metabolism is the whole of the biochemical processes responsible for the metabolic formation, breakdown, and interconversion of carbohydrates in living organisms.
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.
The term amphibolism is used to describe a biochemical pathway that involves both catabolism and anabolism. Catabolism is a degradative phase of metabolism in which large molecules are converted into smaller and simpler molecules, which involves two types of reactions. First, hydrolysis reactions, in which catabolism is the breaking apart of molecules into smaller molecules to release energy. Examples of catabolic reactions are digestion and cellular respiration, where sugars and fats are broken down for energy. Breaking down a protein into amino acids, or a triglyceride into fatty acids, or a disaccharide into monosaccharides are all hydrolysis or catabolic reactions. Second, oxidation reactions involve the removal of hydrogens and electrons from an organic molecule. Anabolism is the biosynthesis phase of metabolism in which smaller simple precursors are converted to large and complex molecules of the cell. Anabolism has two classes of reactions. The first are dehydration synthesis reactions; these involve the joining of smaller molecules together to form larger, more complex molecules. These include the formation of carbohydrates, proteins, lipids and nucleic acids. The second are reduction reactions, in which hydrogens and electrons are added to a molecule. Whenever that is done, molecules gain energy.

Chemiosmosis is the movement of ions across a semipermeable membrane bound structure, down their electrochemical gradient. An important example is the formation of adenosine triphosphate (ATP) by the movement of hydrogen ions (H+) across a membrane during cellular respiration or photosynthesis.

In the mitochondrion, the matrix is the space within the inner membrane. The word "matrix" stems from the fact that this space is viscous, compared to the relatively aqueous cytoplasm. The mitochondrial matrix contains the mitochondrial DNA, ribosomes, soluble enzymes, small organic molecules, nucleotide cofactors, and inorganic ions.[1] The enzymes in the matrix facilitate reactions responsible for the production of ATP, such as the citric acid cycle, oxidative phosphorylation, oxidation of pyruvate, and the beta oxidation of fatty acids.
Bioenergetics is a field in biochemistry and cell biology that concerns energy flow through living systems. This is an active area of biological research that includes the study of the transformation of energy in living organisms and the study of thousands of different cellular processes such as cellular respiration and the many other metabolic and enzymatic processes that lead to production and utilization of energy in forms such as adenosine triphosphate (ATP) molecules. That is, the goal of bioenergetics is to describe how living organisms acquire and transform energy in order to perform biological work. The study of metabolic pathways is thus essential to bioenergetics.
Substrate-level phosphorylation is a metabolism reaction that results in the production of ATP or GTP supported by the energy released from another high-energy bond that leads to phosphorylation of ADP or GDP to ATP or GTP (note that the reaction catalyzed by creatine kinase is not considered as "substrate-level phosphorylation"). This process uses some of the released chemical energy, the Gibbs free energy, to transfer a phosphoryl (PO3) group to ADP or GDP. Occurs in glycolysis and in the citric acid cycle.
The Pasteur effect describes how available oxygen inhibits ethanol fermentation, driving yeast to switch toward aerobic respiration for increased generation of the energy carrier adenosine triphosphate (ATP). More generally, in the medical literature, the Pasteur effect refers to how the cellular presence of oxygen causes in cells a decrease in the rate of glycolysis and also a suppression of lactate accumulation. The effect occurs in animal tissues, as well as in microorganisms belonging to the fungal kingdom.

Bioenergetic systems are metabolic processes that relate to the flow of energy in living organisms. Those processes convert energy into adenosine triphosphate (ATP), which is the form suitable for muscular activity. There are two main forms of synthesis of ATP: aerobic, which uses oxygen from the bloodstream, and anaerobic, which does not. Bioenergetics is the field of biology that studies bioenergetic systems.
Cellular waste products are formed as a by-product of cellular respiration, a series of processes and reactions that generate energy for the cell, in the form of ATP. One example of cellular respiration creating cellular waste products are aerobic respiration and anaerobic respiration.
Pseudohypoxia refers to a condition that mimics hypoxia, by having sufficient oxygen yet impaired mitochondrial respiration due to a deficiency of necessary co-enzymes, such as NAD+ and TPP. The increased cytosolic ratio of free NADH/NAD+ in cells (more NADH than NAD+) can be caused by diabetic hyperglycemia and by excessive alcohol consumption. Low levels of TPP results from thiamine deficiency.