A borane is a compound with the formula BxHy or a related anion. Many such boranes are known. Most common are those with 1 to 12 boron atoms. Although they have few practical applications, the boranes exhibit structures and bonding that differs strongly from the patterns seen in hydrocarbons. Hybrids of boranes and hydrocarbons, the carboranes are also well developed.

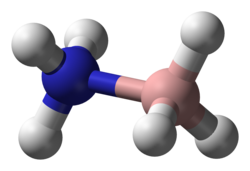
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane () is a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis.

Diborane(6), commonly known as diborane, is the chemical compound with the formula B2H6. It is a toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents.
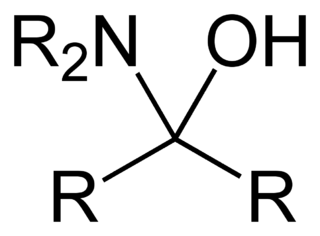
In organic chemistry, a hemiaminal is a functional group or type of chemical compound that has a hydroxyl group and an amine attached to the same carbon atom: −C(OH)(NR2)−. R can be hydrogen or an alkyl group. Hemiaminals are intermediates in imine formation from an amine and a carbonyl by alkylimino-de-oxo-bisubstitution. Hemiaminals can be viewed as a blend of aminals and geminal diol. They are a special case of amino alcohols.

Organoboron chemistry or organoborane chemistry studies organoboron compounds, also called organoboranes. These chemical compounds combine boron and carbon; typically, they are organic derivatives of borane (BH3), as in the trialkyl boranes.
In organic chemistry, hydroboration refers to the addition of a hydrogen-boron bond to certain double and triple bonds involving carbon. This chemical reaction is useful in the organic synthesis of organic compounds.
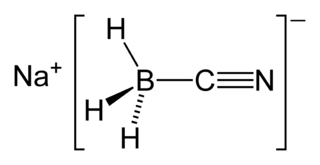
Sodium cyanoborohydride is a chemical compound with the formula Na[BH3(CN)]. It is a colourless salt used in organic synthesis for chemical reduction including that of imines and carbonyls. Sodium cyanoborohydride is a milder reductant than other conventional reducing agents.

n-Butylamine is an organic compound (specifically, an amine) with the formula CH3(CH2)3NH2. This colourless liquid is one of the four isomeric amines of butane, the others being sec-butylamine, tert-butylamine, and isobutylamine. It is a liquid having the fishy, ammonia-like odor common to amines. The liquid acquires a yellow color upon storage in air. It is soluble in all organic solvents. Its vapours are heavier than air and it produces toxic oxides of nitrogen during combustion.

tert-Butylamine (also erbumine and other names) is an organic chemical compound with the formula (CH3)3CNH2. It is a colorless liquid with a typical amine-like odor. tert-Butylamine is one of the four isomeric amines of butane, the others being n-butylamine, sec-butylamine and isobutylamine.
A frustrated Lewis pair (FLP) is a compound or mixture containing a Lewis acid and a Lewis base that, because of steric hindrance, cannot combine to form a classical adduct. Many kinds of FLPs have been devised, and many simple substrates exhibit activation.

Borane dimethylsulfide (BMS) is a chemical compound with the chemical formula BH3·S(CH3)2. It is an adduct between borane molecule and dimethyl sulfide molecule. It is a complexed borane reagent that is used for hydroborations and reductions. The advantages of BMS over other borane reagents, such as borane-tetrahydrofuran, are its increased stability and higher solubility. BMS is commercially available at much higher concentrations than its tetrahydrofuran counterpart and does not require sodium borohydride as a stabilizer, which could result in undesired side reactions. In contrast, BH3·THF requires sodium borohydride to inhibit reduction of THF to tributyl borate. BMS is soluble in most aprotic solvents.

Borane–tetrahydrofuran is an adduct derived from borane and tetrahydrofuran (THF). These solutions, which are colorless, are used for reductions and hydroboration, reactions that are useful in synthesis of organic compounds. The use of borane–tetrahydrofuran has been displaced by borane–dimethylsulfide, which has a longer shelf life and effects similar transformations.
Dehydrogenation of amine-boranes or dehydrocoupling of amine-boranes is a chemical process in main group and organometallic chemistry wherein dihydrogen is released by the coupling of two or more amine-borane adducts. This process is of due to the potential of using amine-boranes for hydrogen storage.
Borane, also known as borine, is an unstable and highly reactive molecule with the chemical formula BH
3. The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated the likely existence of the borane molecule. However, the molecular species BH3 is a very strong Lewis acid. Consequently, it is highly reactive and can only be observed directly as a continuously produced, transitory, product in a flow system or from the reaction of laser ablated atomic boron with hydrogen. It normally dimerizes to diborane in the absence of other chemicals.

Borinic acid, also known as boronous acid, is an oxyacid of boron with formula H
2BOH. Borinate is the associated anion of borinic acid with formula H
2BO−
; however, being a Lewis acid, the form in basic solution is H
2B(OH)−
2.

1,2-Dimethyldiborane is an organoboron compound with the formula [(CH3)BH2]2. Structurally, it is related to diborane, but with methyl groups replacing terminal hydrides on each boron. It is the dimer of methylborane, CH3BH2, the simplest alkylborane. 1,2-Dimethyldiborane can exist in a cis- and a trans arrangement. 1,2-Dimethyldiborane is an easily condensed, colorless gas that ignites spontaneously in air.
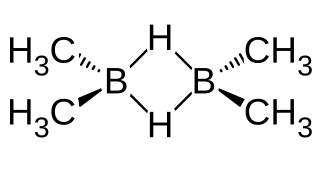
Dimethylborane, (CH3)2BH is the simplest dialkylborane, consisting of a methyl group substituted for a hydrogen in borane. As for other boranes it normally exists in the form of a dimer called tetramethyldiborane or tetramethylbisborane or TMDB ((CH3)2BH)2. Other combinations of methylation occur on diborane, including monomethyldiborane, trimethyldiborane, 1,2-dimethylborane, 1,1-dimethylborane and trimethylborane. At room temperature the substance is at equilibrium between these forms. The methylboranes were first prepared by H. I. Schlesinger and A. O. Walker in the 1930s.

Trimethyldiborane, (CH3)3B2H3 is a molecule containing boron carbon and hydrogen. It is an alkylborane, consisting of three methyl group substituted for a hydrogen in diborane. It can be considered a mixed dimer: (CH3)2BH2BH(CH3) or dimethylborane and methylborane. called 1,2-dimethyldiborane. Other combinations of methylation occur on diborane, including monomethyldiborane, 1,2-dimethyldiborane, tetramethyldiborane, 1,1-dimethylborane and trimethylborane. At room temperature the substance is at equilibrium between these forms, so it is difficult to keep it pure. The methylboranes were first prepared by H. I. Schlesinger and A. O. Walker in the 1930s.
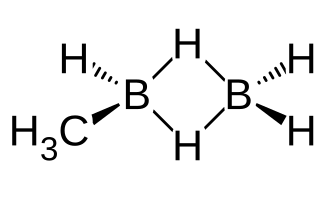
Methyldiborane, CH3B2H5, or monomethyldiborane is the simplest of alkyldiboranes, consisting of a methyl group substituted for a hydrogen in diborane. As with other boranes it exists in the form of a dimer with a twin hydrogen bridge that uses three-center two-electron bonding between the two boron atoms, and can be imagined as methyl borane (CH3BH2) bound to borane (BH3). Other combinations of methylation occur on diborane, including 1,1-dimethylborane, 1,2-dimethyldiborane, trimethyldiborane, tetramethyldiborane, and trimethylborane (which is not a dimer). At room temperature the substance is at equilibrium between these molecules.

Borane carbonyl is the inorganic compound with the formula H3BCO. This colorless gas is the adduct of borane and carbon monoxide. It is usually prepared by combining borane-ether complexes and CO. The compound is mainly of theoretical and pedagogical interest.