Related Research Articles

The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element.

Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids (DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere.
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate BO3−3, metaborate BO−2, or tetraborate B4O2−7; or any salt of such anions, such as sodium metaborate, Na+[BO2]− and borax (Na+)2[B4O7]2−. The name also refers to esters of such anions, such as trimethyl borate B(OCH3)3.

An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as 100 °C (212 °F). While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ionic liquids are largely made of ions. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses.
Boron trifluoride is the inorganic compound with the formula BF3. This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.

Potassium superoxide is an inorganic compound with the formula KO2. It is a yellow paramagnetic solid that decomposes in moist air. It is a rare example of a stable salt of the superoxide anion. It is used as a CO
2 scrubber, H
2O dehumidifier, and O
2 generator in rebreathers, spacecraft, submarines, and spacesuits.

Ozonide is the polyatomic anion O−3. Cyclic organic compounds formed by the addition of ozone to an alkene are also called ozonides.

Tetrafluoroborate is the anion BF−
4. This tetrahedral species is isoelectronic with tetrafluoroberyllate (BeF2−
4), tetrafluoromethane (CF4), and tetrafluoroammonium (NF+
4) and is valence isoelectronic with many stable and important species including the perchlorate anion, ClO−
4, which is used in similar ways in the laboratory. It arises by the reaction of fluoride salts with the Lewis acid BF3, treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.
Molten salt is salt which is solid at standard temperature and pressure but has become liquid due to elevated temperature. Regular table salt has a melting point of 801 °C (1474 °F) and a heat of fusion of 520 J/g.

The tetrafluoroammonium cation is a positively charged polyatomic ion with chemical formula NF+
4. It is equivalent to the ammonium ion where the hydrogen atoms surrounding the central nitrogen atom have been replaced by fluorine. Tetrafluoroammonium ion is isoelectronic with tetrafluoromethane CF
4, trifluoramine oxide ONF
3 and the tetrafluoroborate BF−
4 anion.
Perchloratoborate is an anion of the form [B(ClO4)4]−. It can form partly stable solid salts with heavy alkali metals. They are more stable than nitratoborate salts. K[B(ClO4)4] decomposes at 35 °C, Rb[B(ClO4)4] is stable to 50 °C, and Cs[B(ClO4)4] can exist up to 80 °C.
Tetraperchloratoaluminates are salts of the tetraperchloratoaluminate anion, [Al(ClO4)4]−. The anion contains aluminium tetrahedrally surrounded by four perchlorate groups. The perchlorate is covalently bonded to the aluminium, but perchlorate is much more well known as an ion. The covalent bond to aluminium distorts the perchlorate and renders it unstable.
Pentanitratoaluminate is an anion of aluminium and nitrate groups with formula [Al(NO3)5]2− that can form salts called pentanitratoaluminates. It is unusual being a complex with five nitrate groups, and being a nitrate complex of a light element with nitrate. Such a complex with five nitrate groups is called a pentanitratometallate.
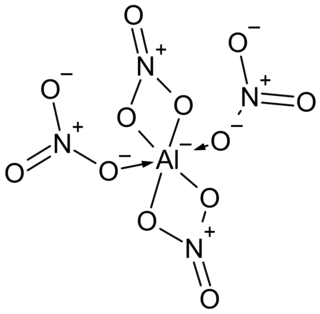
Tetranitratoaluminate is an anion of aluminium and nitrate groups with formula [Al(NO3)4]− that can form salts called tetranitratoaluminates. It is unusual in being a nitrate complex of a light element.
Hexanitratoaluminate is an anion of aluminium and six nitrate groups with formula [Al(NO3)6]3− that can form salts called hexanitratoaluminates.
Zirconium perchlorate is a molecular substance containing zirconium and perchlorate groups with formula Zr(ClO4)4. Zr(ClO4)4 is a volatile crystalline product. It can be formed by reacting zirconium tetrachloride with dry perchloric acid at liquid nitrogen temperatures. Zr(ClO4)4 sublimes slowly in a vacuum at 70°C showing that the molecule is covalently bound rather than being ionic. The reaction also forms some zirconyl perchlorate (or zirconium oxyperchlorate) ZrO(ClO4)2 as even apparently pure perchloric acid is in equilibrium with dichlorine heptoxide, hydronium ions and perchlorate ions. This side product can be minimised by adding more dichlorine heptoxide or doing the reaction as cold as possible.
The hexaperchloratoaluminate ion is a polyatomic anion with the chemical formula [Al(ClO4)6]3−. It is composed of six perchlorate (ClO−4) anions bound to a central aluminium ion (Al3+), resulting in a net charge of –3. This ion is a highly oxidizing and reactive complex, similar to other hexacoordinate aluminium complexes such as hexanitratoaluminate.

Caesium ozonide (CsO3) is an oxygen-rich compound of caesium. It is an ozonide, meaning it contains the ozonide anion (O3−). It can be formed by reacting ozone with caesium superoxide:

Rubidium ozonide is an oxygen rich compound of rubidium. It is an ozonide, meaning it contains the ozonide anion (O3−).
References
- 1 2 3 4 5 6 Guibert, C. R.; M. D. Marshall (1966). "Synthesis of the Tetranitratoborate Anion". Journal of the American Chemical Society. 88 (1): 189–190. doi:10.1021/ja00953a051. ISSN 0002-7863.
- 1 2 3 4 Titova, K. V.; V. Ya. Rosolovskii (1970). "Tetraalkylammonium nitratoborates". Bulletin of the Academy of Sciences of the USSR Division of Chemical Science. 19 (12): 2515–2519. doi:10.1007/BF00854900. ISSN 0568-5230.
- ↑ Jones, CJ Bigler (2007). Transition and Main Group Metals Applied to Oxidative Functionalization of Methane and Use as High Oxygen Carriers for Rocket Propellants. p. 139. ISBN 9780549231066 . Retrieved 3 February 2014.
- 1 2 Titova, K. V.; V. Ya. Rosolovskii (1975). "Reaction of nitrates of monovalent cations with BCl3". Bulletin of the Academy of Sciences of the USSR Division of Chemical Science. 24 (10): 2246–2248. doi:10.1007/BF00929774. ISSN 0568-5230.
- ↑ C.C. Addison; D. Sutton. Progress in Inorganic Chemistry. Vol. 8. p. 216.
- ↑ Jones, C. Bigler; Ralf Haiges; Thorsten Schroer; Karl O. Christe (2006). "Oxygen-Balanced Energetic Ionic Liquid". Angewandte Chemie International Edition. 45 (30): 4981–4984. doi:10.1002/anie.200600735. ISSN 1433-7851. PMID 16819744.
| HNO3 | He | |||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO3)−4 | RONO2 | NO−3 NH4NO3 | HOONO2 | FNO3 | Ne | |||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 Al(NO3)−4 | Si | P | S | ClONO2 | Ar | |||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)2 Fe(NO3)3 | Co(NO3)2 Co(NO3)3 | Ni(NO3)2 | CuNO3 Cu(NO3)2 | Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | BrNO3 | Kr | |
| RbNO3 | Sr(NO3)2 | Y(NO3)3 | Zr(NO3)4 | NbO(NO3)3 | MoO2(NO3)2 | Tc | Ru(NO3)3 | Rh(NO3)3 | Pd(NO3)2 Pd(NO3)4 | AgNO3 Ag(NO3)2 | Cd(NO3)2 | In(NO3)3 | Sn(NO3)4 | Sb(NO3)3 | Te | INO3 | Xe(NO3)2 | |
| CsNO3 | Ba(NO3)2 | Lu(NO3)3 | Hf(NO3)4 | TaO(NO3)3 | W | Re | Os | Ir | Pt(NO3)2 Pt(NO3)4 | Au(NO3)3 | Hg2(NO3)2 Hg(NO3)2 | TlNO3 Tl(NO3)3 | Pb(NO3)2 | Bi(NO3)3 BiO(NO3) | Po(NO3)4 | At | Rn | |
| FrNO3 | Ra(NO3)2 | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | ||||||||||||||||||
| La(NO3)3 | Ce(NO3)3 Ce(NO3)4 | Pr(NO3)3 | Nd(NO3)3 | Pm(NO3)3 | Sm(NO3)3 | Eu(NO3)3 | Gd(NO3)3 | Tb(NO3)3 | Dy(NO3)3 | Ho(NO3)3 | Er(NO3)3 | Tm(NO3)3 | Yb(NO3)3 | |||||
| Ac(NO3)3 | Th(NO3)4 | PaO2(NO3)3 | UO2(NO3)2 | Np(NO3)4 | Pu(NO3)4 | Am(NO3)3 | Cm(NO3)3 | Bk(NO3)3 | Cf | Es | Fm | Md | No | |||||